- Record: found
- Abstract: found
- Article: found
Cancer-Associated Fibroblasts from Hepatocellular Carcinoma Promote Malignant Cell Proliferation by HGF Secretion
Read this article at
Abstract
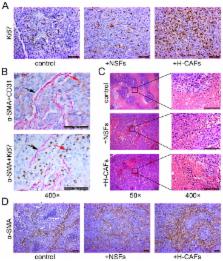
Cancer-associated fibroblasts (CAFs) are reported to support tumorigenesis by stimulating angiogenesis, cancer cell proliferation, and invasion in most solid tumors. However, the roles of CAFs in the liver cancer microenvironment have not been thoroughly studied. In our previous study, we successfully isolated CAFs from hepatocellular carcinoma (HCC) (H-CAFs) and proved that H-CAFs suppressed the activation of NK cells and thereby created favorable conditions for HCC progression. In our present study, we found that the proliferation of MHCC97L and Hep3B cells was significantly promoted by treatment with conditioned medium from H-CAFs. Pathological analysis also revealed that H-CAFs increased the proportion of Ki-67 (+) malignant cells and prevented them from undergoing necrosis. Moreover, the concentration of hepatocyte growth factor (HGF) cytokine in the conditioned medium of H-CAFs was higher than conditioned medium from normal skin fibroblasts (NSFs). Anti-HGF significantly reduced the proliferation-promoting capability of H-CAFs. In addition, we found that the abundance of H-CAFs correlated positively with tumor size. These results indicate that H-CAFs are an important factor for promoting the growth of HCC in vitro and in vivo, and that HGF plays a key role in HCC proliferation induced by H-CAFs.
Related collections
Most cited references20
- Record: found
- Abstract: found
- Article: not found
Cancer-associated stromal fibroblasts promote pancreatic tumor progression.

- Record: found
- Abstract: found
- Article: found
Cancer Associated Fibroblasts Promote Tumor Growth and Metastasis by Modulating the Tumor Immune Microenvironment in a 4T1 Murine Breast Cancer Model
- Record: found
- Abstract: found
- Article: not found