- Record: found
- Abstract: found
- Article: found
Inhibition of p38 Mitogen-Activated Protein Kinase Impairs Mayaro Virus Replication in Human Dermal Fibroblasts and HeLa Cells
Read this article at
Abstract
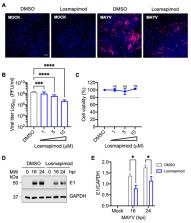
Mayaro virus (MAYV) hijacks the host’s cell machinery to effectively replicate. The mitogen-activated protein kinases (MAPKs) p38, JNK, and ERK1/2 have emerged as crucial cellular factors implicated in different stages of the viral cycle. However, whether MAYV uses these MAPKs to competently replicate has not yet been determined. The aim of this study was to evaluate the impact of MAPK inhibition on MAYV replication using primary human dermal fibroblasts (HDFs) and HeLa cells. Viral yields in supernatants from MAYV-infected cells treated or untreated with inhibitors SB203580, SP600125, U0126, or Losmapimod were quantified using plaque assay. Additionally, viral protein expression was analyzed using immunoblot and immunofluorescence. Knockdown of p38⍺/p38β isoforms was performed in HDFs using the PROTACs molecule NR-7h. Our data demonstrated that HDFs are highly susceptible to MAYV infection. SB203580, a p38 inhibitor, reduced MAYV replication in a dose-dependent manner in both HDFs and HeLa cells. Additionally, SB203580 significantly decreased viral E1 protein expression. Similarly, knockdown or inhibition of p38⍺/p38β isoforms with NR-7h or Losmapimod, respectively, affected MAYV replication in a dose-dependent manner. Collectively, these findings suggest that p38 could play an important role in MAYV replication and could serve as a therapeutic target to control MAYV infection.
Related collections
Most cited references59
- Record: found
- Abstract: found
- Article: not found
Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method.
- Record: found
- Abstract: found
- Article: not found
The Global Phosphorylation Landscape of SARS-CoV-2 Infection
- Record: found
- Abstract: found
- Article: not found