- Record: found
- Abstract: found
- Article: found
Biomphalysin, a New β Pore-forming Toxin Involved in Biomphalaria glabrata Immune Defense against Schistosoma mansoni
Read this article at
Abstract
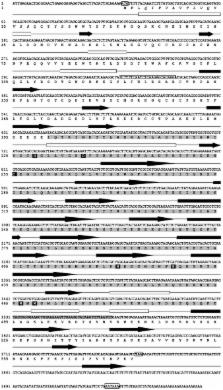
Aerolysins are virulence factors belonging to the β pore-forming toxin (β-PFT) superfamily that are abundantly distributed in bacteria. More rarely, β-PFTs have been described in eukaryotic organisms. Recently, we identified a putative cytolytic protein in the snail, Biomphalaria glabrata, whose primary structural features suggest that it could belong to this β-PFT superfamily. In the present paper, we report the molecular cloning and functional characterization of this protein, which we call Biomphalysin, and demonstrate that it is indeed a new eukaryotic β-PFT. We show that, despite weak sequence similarities with aerolysins, Biomphalysin shares a common architecture with proteins belonging to this superfamily. A phylogenetic approach revealed that the gene encoding Biomphalysin could have resulted from horizontal transfer. Its expression is restricted to immune-competent cells and is not induced by parasite challenge. Recombinant Biomphalysin showed hemolytic activity that was greatly enhanced by the plasma compartment of B. glabrata. We further demonstrated that Biomphalysin with plasma is highly toxic toward Schistosoma mansoni sporocysts. Using in vitro binding assays in conjunction with Western blot and immunocytochemistry analyses, we also showed that Biomphalysin binds to parasite membranes. Finally, we showed that, in contrast to what has been reported for most other members of the family, lytic activity of Biomphalysin is not dependent on proteolytic processing. These results provide the first functional description of a mollusk immune effector protein involved in killing S. mansoni.
Author Summary
Schistosomiasis is the second most widespread tropical parasitic disease after malaria. It is caused by flatworms of the genus Schistosoma. Its life cycle is complex and requires certain freshwater snail species as intermediate host. Given the limited options for treating S. mansoni infections, much research has focused on a better understanding of the immunobiological interactions between the invertebrate host Biomphalaria glabrata and its parasite S. mansoni. A number of studies published over the last two decades have contributed greatly to our understanding of B. glabrata innate immune mechanisms involved in the defense against parasite. However, most studies have focused on the identification of recognition molecules or immune receptors involved in the host/parasite interplay. In the present study, we report the first functional description of a mollusk immune effector protein involved in killing S. mansoni, a protein related to the β pore forming toxin that we named Biomphalysin.
Related collections
Most cited references58
- Record: found
- Abstract: found
- Article: not found
ProtTest: selection of best-fit models of protein evolution.
- Record: found
- Abstract: found
- Article: not found
A family of fibrinogen-related proteins that precipitates parasite-derived molecules is produced by an invertebrate after infection.
- Record: found
- Abstract: found
- Article: not found