- Record: found
- Abstract: found
- Article: found
LPS Primes Brain Responsiveness to High Mobility Group Box-1 Protein
Read this article at
Abstract
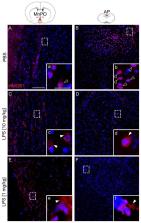
High mobility group box (HMGB)1 action contributes to late phases of sepsis, but the effects of increased endogenous plasma HMGB1 levels on brain cells during inflammation are unclear. Here, we aimed to further investigate the role of HMGB1 in the brain during septic-like lipopolysaccharide-induced inflammation in rats (LPS, 10 mg/kg, i.p.). HMGB-1 mRNA expression and release were measured in the periphery/brain by RT-PCR, immunohistochemistry and ELISA. In vitro experiments with disulfide-HMGB1 in primary neuro-glial cell cultures of the area postrema (AP), a circumventricular organ with a leaky blood–brain barrier and direct access to circulating mediators like HMGB1 and LPS, were performed to determine the direct influence of HMGB1 on this pivotal brain structure for immune-to-brain communication. Indeed, HMGB1 plasma levels stayed elevated after LPS injection. Immunohistochemistry of brains and AP cultures confirmed LPS-stimulated cytoplasmatic translocation of HMGB1 indicative of local HMGB1 release. Moreover, disulfide-HMGB1 stimulation induced nuclear factor (NF)-κB activation and a significant release of interleukin-6, but not tumor necrosis factor α, into AP culture supernatants. However, only a few AP cells directly responded to HMGB1 with increased intracellular calcium concentration. Interestingly, priming with LPS induced a seven-fold higher percentage of responsive cells to HMGB1. We conclude that, as a humoral and local mediator, HMGB1 enhances brain inflammatory responses, after LPS priming, linked to sustained sepsis symptoms.
Related collections
Most cited references106
- Record: found
- Abstract: found
- Article: not found
HMG-1 as a late mediator of endotoxin lethality in mice.
- Record: found
- Abstract: found
- Article: not found
Polyunsaturated fatty acids and their metabolites in brain function and disease.
- Record: found
- Abstract: found
- Article: not found