- Record: found
- Abstract: found
- Article: found
The efficacy and safety of dupilumab in Chinese patients with moderate‐to‐severe atopic dermatitis: a randomized, double‐blind, placebo‐controlled study*
Read this article at
Summary
Background
Dupilumab is an antibody against interleukin‐4 receptor α, used in the treatment of atopic dermatitis (AD).
Objectives
To evaluate the efficacy and safety of dupilumab in adult Chinese patients with moderate‐to‐severe AD.
Methods
In this randomized, double‐blind, placebo‐controlled, parallel‐group, phase III study, conducted between December 2018 and February 2020, patients with AD received dupilumab (300 mg) or placebo once every 2 weeks for 16 weeks, and were followed up for 12 weeks. The primary efficacy endpoint was the proportion of patients with both an Investigator’s Global Assessment score of 0–1 and a reduction from baseline of ≥ 2 points at week 16.
Results
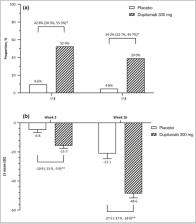
Overall, 165 patients (mean age 30·6 years; 71·5% male patients) were randomized; 82 patients were randomized to dupilumab and 83 patients were randomized to placebo. At week 16, 26·8% of patients in the dupilumab group and 4·8% of patients in the placebo group achieved the primary endpoint [difference 22·0%, 95% confidence interval (CI) 11·37–32·65; P < 0·001]. Compared with placebo, higher proportions of patients in the dupilumab group achieved ≥ 75% reduction in the Eczema Area and Severity Index score (57·3% vs. 14·5%; difference 42·9%, 95% CI 29·75–55·97; P < 0·001) and had ≥ 3‐point (52·4% vs. 9·6%; difference 42·8%, 95% CI 30·26–55·34; P < 0·001) and ≥ 4‐point (39·0% vs. 4·8%; difference 34·2%, 95% CI 22·69–45·72; P < 0·001) reductions in weekly average daily peak daily pruritus numerical rating scale scores. The incidence of treatment‐emergent adverse events during the treatment period was similar in the two groups. The incidence of conjunctivitis, allergic conjunctivitis and injection site reaction was higher in the dupilumab group than in the placebo group.
Abstract
What is already known about this topic?
-
Two randomized, placebo‐controlled phase III trials (SOLO 1 and SOLO 2) have demonstrated that dupilumab is effective and safe when used in the treatment of atopic dermatitis in adults.
-
However, very few Chinese participants were included and, therefore, the efficacy and safety of dupilumab in this population is unclear.
What does this study add?
-
The findings of this randomized, placebo‐controlled phase III trial show that dupilumab is effective in improving the signs and symptoms of atopic dermatitis in adult Chinese patients and has a favourable safety profile in this population.
Plain language summary available online
Related collections
Most cited references17
- Record: found
- Abstract: found
- Article: not found
Two Phase 3 Trials of Dupilumab versus Placebo in Atopic Dermatitis.
- Record: found
- Abstract: found
- Article: not found