- Record: found
- Abstract: found
- Article: found
Nrf2 drives oxidative stress-induced autophagy in nucleus pulposus cells via a Keap1/Nrf2/p62 feedback loop to protect intervertebral disc from degeneration
Read this article at
Abstract
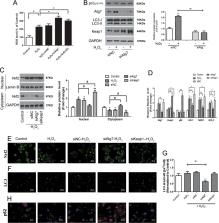
Intervertebral disc (IVD) degeneration is known to aggravate with age and oxidative stress is implicated in the pathogenesis of many age-related diseases. Nuclear factor (erythroid-derived-2)-like 2 (Nrf2) can confer adaptive protection against oxidative and proteotoxic stress in cells. In this study, we assessed whether Nrf2 can protect against oxidative stress in nucleus pulposus (NP) cells. In addition, we investigated Nrf2 expression in NP tissue samples from patients with different degrees of IVD degeneration and a mouse model of aging and IVD degeneration and the influence of H 2O 2-induced oxidative stress on autophagic pathways in NP cells. Autophagy was assessed by measuring levels of autophagy-related protein (ATG) family members and the autophagic markers, p62 and LC3. We found that expression of Nrf2 progressively decreased in human NP tissue samples of patients with increasing degrees of IVD degeneration. Nrf2 deficiency leads to the degeneration of IVDs during aging. Nrf2 knockout also aggravates IVD degeneration and reduces autophagic gene expression in an induced mouse model of IVD degeneration. The detrimental effects of H 2O 2-induced oxidative stress were increased in autophagy-deficient cells via reduced expression of Atg7 and the Keap1–Nrf2–p62 autophagy pathway. Taken together, these results suggest that excessive oxidative stress causes the upregulation of autophagy, and autophagy acts as an antioxidant feedback response activated by a Keap1-Nrf2-p62 feedback loop in IVD degeneration.
Related collections
Most cited references24
- Record: found
- Abstract: found
- Article: not found
Molecular mechanisms of the Keap1–Nrf2 pathway in stress response and cancer evolution.
- Record: found
- Abstract: found
- Article: not found
The Atg12-Atg5 conjugate has a novel E3-like activity for protein lipidation in autophagy.
- Record: found
- Abstract: found
- Article: not found