- Record: found
- Abstract: found
- Article: found
Concise Review: Laying the Groundwork for a First‐In‐Human Study of an Induced Pluripotent Stem Cell‐Based Intervention for Spinal Cord Injury
Read this article at
Abstract
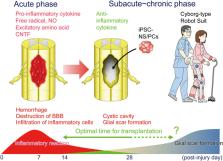
There have been numerous attempts to develop stem cell transplantation approaches to promote the regeneration of spinal cord injury (SCI). Our multicenter team is currently planning to launch a first‐in‐human clinical study of an induced pluripotent stem cell (iPSC)‐based cell transplant intervention for subacute SCI. This trial was conducted as class I regenerative medicine protocol as provided for under Japan's Act on the Safety of Regenerative Medicine, using neural stem/progenitor cells derived from a clinical‐grade, integration‐free human “iPSC stock” generated by the Kyoto University Center for iPS Cell Research and Application. In the present article, we describe how we are preparing to initiate this clinical study, including addressing the issues of safety and tumorigenesis as well as practical problems that must be overcome to enable the development of therapeutic interventions for patients with chronic SCI. stem cells 2019;37:6–13
Abstract
Ash1l can epigenetically promote the expression of essential osteogenic and chondrogenic transcription factors in C3H10T1/2 cells. It exerts this impact via modifications in the enrichment of H3K4me3 on their promoter regions. Ash1l hampered adipogenesis by enhancing H3K4me3 enrichment in promoter of Creb gene, which was reported to be a repressive gene of PPARγ.
Related collections
Most cited references55
- Record: found
- Abstract: found
- Article: not found
Variation in the safety of induced pluripotent stem cell lines.
- Record: found
- Abstract: found
- Article: not found
Direct reprogramming of mouse fibroblasts to neural progenitors.
- Record: found
- Abstract: found
- Article: not found
