- Record: found
- Abstract: found
- Article: found
STAT5 deletion in macrophages alters ductal elongation and branching during mammary gland development
Read this article at
Abstract
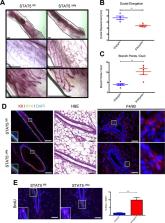
Macrophages are required for proper mammary gland development and maintaining tissue homeostasis. However, the mechanisms by which macrophages regulate this process remain unclear. Here, we identify STAT5 as an important regulator of macrophage function in the developing mammary gland. Analysis of mammary glands from mice with STAT5-deficient macrophages demonstrates delayed ductal elongation, enhanced ductal branching and increased epithelial proliferation. Further analysis reveals that STAT5 deletion in macrophages leads to enhanced expression of proliferative factors such as Cyp19a1/aromatase and IL-6. Mechanistic studies demonstrate that STAT5 binds directly to the Cyp19a1 promoter in macrophages to suppress gene expression and that loss of STAT5 results in enhanced stromal expression of aromatase. Finally, we demonstrate that STAT5 deletion in macrophages cooperates with oncogenic initiation in mammary epithelium to accelerate the formation of estrogen receptor (ER)-positive hyperplasias. These studies establish the importance of STAT5 in macrophages during ductal morphogenesis in the mammary gland and demonstrate that altering STAT5 function in macrophages can affect the development of tissue-specific disease.
Related collections
Most cited references73
- Record: found
- Abstract: found
- Article: not found
Tissue-resident macrophages.
- Record: found
- Abstract: found
- Article: not found
The JAK2/STAT3 signaling pathway is required for growth of CD44⁺CD24⁻ stem cell-like breast cancer cells in human tumors.
- Record: found
- Abstract: found
- Article: not found