- Record: found
- Abstract: found
- Article: found
A Novel B7-H6–Targeted IgG-Like T Cell–Engaging Antibody for the Treatment of Gastrointestinal Tumors
Read this article at
Abstract
Purpose:
Advanced-stage gastrointestinal cancers represent a high unmet need requiring new effective therapies. We investigated the antitumor activity of a novel T cell–engaging antibody (B7-H6/CD3 ITE) targeting B7-H6, a tumor-associated antigen that is expressed in gastrointestinal tumors.
Experimental Design:
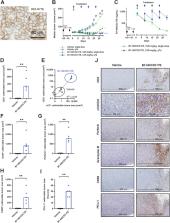
Membrane proteomics and IHC analysis identified B7-H6 as a tumor-associated antigen in gastrointestinal tumor tissues with no to very little expression in normal tissues. The antitumor activity and mode of action of B7-H6/CD3 ITE was evaluated in in vitro coculture assays, in humanized mouse tumor models, and in colorectal cancer precision cut tumor slice cultures.
Results:
B7-H6 expression was detected in 98% of colorectal cancer, 77% of gastric cancer, and 63% of pancreatic cancer tissue samples. B7-H6/CD3 ITE-mediated redirection of T cells toward B7-H6–positive tumor cells resulted in B7-H6–dependent lysis of tumor cells, activation and proliferation of T cells, and cytokine secretion in in vitro coculture assays, and infiltration of T cells into tumor tissues associated with tumor regression in in vivo colorectal cancer models. In primary patient-derived colorectal cancer precision-cut tumor slice cultures, treatment with B7-H6/CD3 ITE elicited cytokine secretion by endogenous tumor-infiltrating immune cells. Combination with anti-PD-1 further enhanced the activity of the B7-H6/CD3 ITE.
Conclusion:
These data highlight the potential of the B7-H6/CD3 ITE to induce T cell–redirected lysis of tumor cells and recruitment of T cells into noninflamed tumor tissues, leading to antitumor activity in in vitro, in vivo, and human tumor slice cultures, which supports further evaluation in a clinical study.
Related collections
Most cited references44
- Record: found
- Abstract: found
- Article: not found
Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients.
- Record: found
- Abstract: found
- Article: not found
The carcinoembryonic antigen (CEA) family: structures, suggested functions and expression in normal and malignant tissues.
- Record: found
- Abstract: not found
- Article: not found