- Record: found
- Abstract: found
- Article: not found
Blood DNA methylation at TXNIP and glycemic changes in response to weight-loss diet interventions: the POUNDS Lost Trial
Read this article at
Abstract
Background:
Thioredoxin Interacting Protein ( TXNIP) functions as a master regulator for glucose homeostasis. Hypomethylation at the 5’-cytosine-phosphate-guanine-3’ (CpG) site cg19693031 of TXNIP has been consistently related to islet dysfunction, hyperglycemia, and type 2 diabetes. DNA methylation (DNAm) may reveal the missing mechanistic link between obesity and type 2 diabetes. We hypothesize that baseline DNAm level at TXNIP in blood may be associated with glycemic traits and their changes in response to weight-loss diet interventions.
Methods:
We included 639 adult participants with overweight or obesity, who participated in a 2-year randomized weight-loss diet intervention. Baseline blood DNAm levels were profiled by high-resolution methylC-capture sequencing. We defined the regional DNAm level of TXNIP as the average methylation level over CpGs within 500 bp of cg19693031. Generalized linear regression models were used for main analyses.
Results:
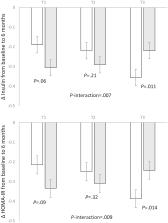
We found that higher regional DNAm at TXNIP was significantly correlated with lower fasting glucose, HbA1c, and Homeostatic Model Assessment of Insulin Resistance (HOMA-IR) at baseline ( P<0.05 for all). Significant interactions were observed between dietary protein intake and DNAm on changes in insulin ( P-interaction=0.007) and HOMA-IR ( P-interaction=0.009) at 6 months. In participants with the highest tertile of regional DNAm at TXNIP, average protein (15%) intake was associated with a greater reduction in insulin (β: −0.14; 95% CI: −0.24, −0.03; P=0.011) and HOMA-IR (β: −0.15; 95% CI: −0.26, −0.03; P=0.014) than high protein (25%) intake, whereas no significant associations were found in those with the lower tertiles ( P>0.05). The interaction was attenuated to be non-significant at 2 years, presumably related to decreasing adherence to the diet intervention.
Related collections
Most cited references40
- Record: found
- Abstract: not found
- Article: not found
Cutadapt removes adapter sequences from high-throughput sequencing reads
- Record: found
- Abstract: found
- Article: not found
Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man.
- Record: found
- Abstract: not found
- Article: not found
