- Record: found
- Abstract: found
- Article: found
Assessment of CYP3A‐mediated drug interaction via cytokine (IL‐6) elevation for mosunetuzumab using physiologically‐based pharmacokinetic modeling
Read this article at
Abstract
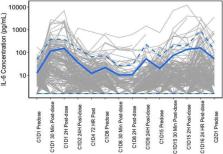
Mosunetuzumab is a CD3/CD20 bispecific antibody. As an on‐target effect, transient elevation of interleukin‐6 (IL‐6) occurs in early treatment cycles. A physiologically‐based pharmacokinetic (PBPK) model was developed to assess potential drug interaction caused by IL‐6 enzyme suppression on cytochrome P450 3A (CYP3A) during mosunetuzumab treatment. The model's performance in predicting IL‐6 CYP3A suppression and subsequent drug–drug interactions (DDIs) was verified using existing clinical data of DDIs caused by chronic and transient IL‐6 elevation. Sensitivity analyses were performed for a complete DDI risk assessment. The IL‐6 concentration‐ and time‐dependent CYP3A suppression during mosunetuzumab treatment was simulated using PBPK model with incorporation of in vitro IL‐6 inhibition data. At clinically approved doses/regimens, the DDI at maximum CYP3A suppression was predicted to be a midazolam maximum drug concentration in plasma (C max) and area under the plasma drug concentration–time curve (AUC) ratio of 1.17 and 1.37, respectively. At the 95th percentile of IL‐6 concentration level or when gut CYP3A suppression was considered, the predicted DDI risk for mosunetuzumab remained low (<2‐fold). The PBPK‐based DDI predictions informed the mosunetuzumab product label to monitor, in early cycles, the concentrations and toxicities for sensitive CYP3A substrates with narrow therapeutic windows.
Related collections
Most cited references34
- Record: found
- Abstract: found
- Article: not found
Clinical pharmacokinetics of therapeutic monoclonal antibodies.
- Record: found
- Abstract: not found
- Article: not found
Safety and efficacy of mosunetuzumab, a bispecific antibody, in patients with relapsed or refractory follicular lymphoma: a single-arm, multicentre, phase 2 study
- Record: found
- Abstract: found
- Article: not found