- Record: found
- Abstract: found
- Article: found
Leishmania donovani development in Phlebotomus argentipes: comparison of promastigote- and amastigote-initiated infections
Read this article at
SUMMARY
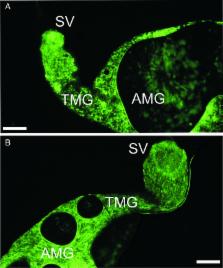
Leishmania parasites alternate in their life cycle between promastigote stages that develop in the gut of phlebotomine sand flies and amastigotes residing inside phagocytic cells of vertebrate hosts. For experimental infections of sand flies, promastigotes are frequently used as this way of infection is technically easier although ingestion of promastigotes by sand flies is unnatural. Here we aimed to answer a critical question, to what extent do promastigote-initiated experimental infections differ from those initiated with intracellular amastigotes. We performed side-by-side comparison of Leishmania development in Phlebotomus argentipes females infected alternatively with promastigotes from log-phase cultures or amastigotes grown ex vivo in macrophages. Early stage infections showed substantial differences in parasite load and representation of morphological forms. The differences disappeared along the maturation of infections; both groups developed heavy late-stage infections with colonization of the stomodeal valve, uniform representation of infective metacyclics and equal efficiency of transmission. The results showed that studies focusing on early phase of Leishmania development in sand flies should be initiated with intracellular amastigotes. However, the use of promastigote stages for sand fly infections does not alter significantly the final outcome of Leishmania donovani development in P. argentipes and their transmissibility to the vertebrate host.
Related collections
Most cited references35
- Record: found
- Abstract: found
- Article: not found
Transmission of Leishmania metacyclic promastigotes by phlebotomine sand flies

- Record: found
- Abstract: found
- Article: found
Leishmania development in sand flies: parasite-vector interactions overview
- Record: found
- Abstract: found
- Article: not found