- Record: found
- Abstract: found
- Article: found
Tissue-specific Tregs in cancer metastasis: opportunities for precision immunotherapy
Read this article at
Abstract
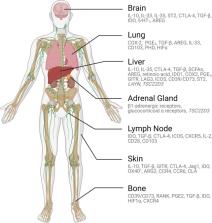
Decades of advancements in immuno-oncology have enabled the development of current immunotherapies, which provide long-term treatment responses in certain metastatic cancer patients. However, cures remain infrequent, and most patients ultimately succumb to treatment-refractory metastatic disease. Recent insights suggest that tumors at certain organ sites exhibit distinctive response patterns to immunotherapy and can even reduce antitumor immunity within anatomically distant tumors, suggesting the activation of tissue-specific immune tolerogenic mechanisms in some cases of therapy resistance. Specialized immune cells known as regulatory T cells (Tregs) are present within all tissues in the body and coordinate the suppression of excessive immune activation to curb autoimmunity and maintain immune homeostasis. Despite the high volume of research on Tregs, the findings have failed to reconcile tissue-specific Treg functions in organs, such as tolerance, tissue repair, and regeneration, with their suppression of local and systemic tumor immunity in the context of immunotherapy resistance. To improve the understanding of how the tissue-specific functions of Tregs impact cancer immunotherapy, we review the specialized role of Tregs in clinically common and challenging organ sites of cancer metastasis, highlight research that describes Treg impacts on tissue-specific and systemic immune regulation in the context of immunotherapy, and summarize ongoing work reporting clinically feasible strategies that combine the specific targeting of Tregs with systemic cancer immunotherapy. Improved knowledge of Tregs in the framework of their tissue-specific biology and clinical sites of organ metastasis will enable more precise targeting of immunotherapy and have profound implications for treating patients with metastatic cancer.
Related collections
Most cited references198
- Record: found
- Abstract: found
- Article: not found
Primary, Adaptive, and Acquired Resistance to Cancer Immunotherapy.
- Record: found
- Abstract: not found
- Article: not found
Five-Year Survival with Combined Nivolumab and Ipilimumab in Advanced Melanoma
- Record: found
- Abstract: found
- Article: not found