- Record: found
- Abstract: found
- Article: found
MAPKs signaling is obligatory for male reproductive function in a development-specific manner
Read this article at
Abstract
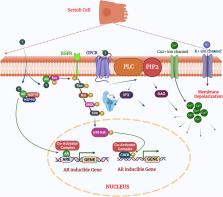
Mitogen-activated protein kinases (MAPKs) represent widely expressed and evolutionarily conserved proteins crucial for governing signaling pathways and playing essential roles in mammalian male reproductive processes. These proteins facilitate the transmission of signals through phosphorylation cascades, regulating diverse intracellular functions encompassing germ cell development in testis, physiological maturation of spermatozoa within the epididymis, and motility regulation at ejaculation in the female reproductive tract. The conservation of these mechanisms appears prevalent across species, including humans, mice, and, to a limited extent, livestock species such as bovines. In Sertoli cells (SCs), MAPK signaling not only regulates the proliferation of immature SCs but also determines the appropriate number of SCs in the testes at puberty, thereby maintaining male fertility by ensuring the capacity for sperm cell production. In germ cells, MAPKs play a crucial role in dynamically regulating testicular cell-cell junctions, supporting germ cell proliferation and differentiation. Throughout spermatogenesis, MAPK signaling ensures the appropriate Sertoli-to-germ cell ratio by regulating apoptosis, controlling the metabolism of developing germ cells, and facilitating the maturation of spermatozoa within the cauda epididymis. During ejaculation in the female reproductive tract, MAPKs regulate two pivotal events—capacitation and the acrosome reaction essential for maintaining the fertility potential of sperm cells. Any disruptions in MAPK pathway signaling possibly may disturb the testicular microenvironment homeostasis, sperm physiology in the male body before ejaculation and in the female reproductive tract during fertilization, ultimately compromising male fertility. Despite decades of research, the physiological function of MAPK pathways in male reproductive health remains inadequately understood. The current review attempts to combine recent findings to elucidate the impact of MAPK signaling on male fertility and proposes future directions to enhance our understanding of male reproductive functions.
Related collections
Most cited references78
- Record: found
- Abstract: found
- Article: not found
Cell signaling by receptor tyrosine kinases.
- Record: found
- Abstract: found
- Article: not found
ROS and ROS-Mediated Cellular Signaling
- Record: found
- Abstract: found
- Article: not found