- Record: found
- Abstract: found
- Article: found
Supplemental Clostridium butyricum Modulates Lipid Metabolism Through Shaping Gut Microbiota and Bile Acid Profile of Aged Laying Hens
Read this article at
Abstract
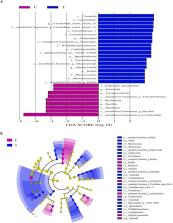
Probiotic Clostridium butyricum could affect lipid metabolism in broilers. However, it is not clear whether C. butyricum could improve lipid metabolism through shaping gut microbiota and bile acid (BA) profile of laying hens. We aimed to evaluate the contributions of gut microbiota and BA profile to the potential effect of C. butyricum on lipid metabolism of aged laying hens. A total of 192 60-week-old Hy-Line Brown laying hens were divided into two groups (eight replicates per group). Birds were fed a basal diet supplemented with 0 or 2.7 g/kg C. butyricum (1.0 × 10 9 CFU/g). Samples were collected at the end of week 8 of the experiment. The results showed elevated ( P < 0.05) concentrations of glucagon-like peptide 1, insulin and thyroid hormones in serum responded to C. butyricum addition, which also decreased ( P < 0.05) hepatic free fatty acids contents, as well as increased ( P < 0.05) the expression of hepatic acyl-CoA oxidase, farnesoid X receptor (FXR) and PPARα. C. butyricum addition increased ( P < 0.05) Bacteroidetes abundance but tended to decrease ( P < 0.10) Firmicutes abundance in the ileum. Besides, C. butyricum addition resulted in higher ( P < 0.05) abundances of Clostridia ( Clostridiales) and Prevotellaceae, concurrent with an increasing trend ( P < 0.10) of Bifidobacteriaceae abundance and decreased the abundances of several harmful bacteria such as Klebsiella ( P < 0.05). Regarding ileal BA profile, there was a reduced ( P < 0.05) content of tauro-α-muricholic acid, increased ( P < 0.05) contents of tauroursodeoxycholic acid and lithocholic acid, along with increasing trends ( P < 0.10) of glycochenodeoxycholic acid and hyodeoxycholic acid contents due to C. butyricum addition, which also increased ( P < 0.05) ileal FXR expression. Collectively, supplemental C. butyricum accelerated hepatic fatty acid oxidation, and shaped gut microbiota and BA profile, thus reducing fat deposition in the liver of aged laying hens.
Related collections
Most cited references47
- Record: found
- Abstract: found
- Article: not found
Targeted disruption of the nuclear receptor FXR/BAR impairs bile acid and lipid homeostasis.
- Record: found
- Abstract: found
- Article: not found
Liver X receptors in lipid signalling and membrane homeostasis
- Record: found
- Abstract: found
- Article: not found