- Record: found
- Abstract: found
- Article: found
LncRNA CCAT2, involving miR-34a/TGF-β1/Smad4 signaling, regulate hepatic stellate cells proliferation
Read this article at
Abstract
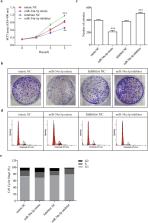
miR-34a targeting on Smad4 plays important role in TGF-β1 pathway which is a dominant factor for balancing collagen production and degradation in hepatic stellate cells. TGF-β1/Smad4 regulated collagen deposition is a hallmark of hepatic fibrosis. The potential regulation on miR-34a by LncRNAs in hepatic stellate cells (HSCs) is still reserved to be revealed. In current study, it was hypothesized that a miR-34a interactor, lncRNA CCAT2 may regulate TGF-β1 pathway in liver fibrotic remodeling. The interaction between CCAT2 and miR-34a-5p was checked by dual luciferase assay. the effects of CCAT2 and miR-34a-5p on cell proliferation and apoptosis were verified by MTT assay, colony formation assay, and flow cytometry assay. Dual luciferase activity showed CCAT2 are targets of miR-34a-5p. Sh-CCAT2 transfection prohibit HSCs proliferation and induce HSCs apoptosis, also inhibited ECM protein synthesis in HSCs. Decreased miR-34a-5p enhanced HSCs proliferation, blocked HSCs apoptosis and promoted ECM protein production. miR-34a-5p inhibitor undo protective regulation of sh-CCAT2 in liver fibrosis. Furthermore, clinical investigation showed that CCAT2 and Smad4 expression level were significantly induced, while miR-34a-5p was significantly decreased in HBV related liver fibrosis serum. In conclusion, activated HSCs via TGF-β1/Smad4 signaling pathway was successfully alleviated by CCAT2 inhibition through miR-34a-5p elevation.
Related collections
Most cited references59
- Record: found
- Abstract: found
- Article: not found
Liver fibrosis.
- Record: found
- Abstract: found
- Article: not found
LncRNA-mediated regulation of cell signaling in cancer
- Record: found
- Abstract: found
- Article: not found