- Record: found
- Abstract: found
- Article: found
Differences in Small Molecule Neurotransmitter Profiles From the Crown-of-Thorns Seastar Radial Nerve Revealed Between Sexes and Following Food-Deprivation
Read this article at
Abstract
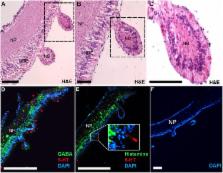
Neurotransmitters serve as chemical mediators of cell communication, and are known to have important roles in regulating numerous physiological and metabolic events in eumetazoans. The Crown-of-Thorns Seastar (COTS) is an asteroid echinoderm that has been the focus of numerous ecological studies due to its negative impact on coral reefs when in large numbers. Research devoted to its neural signaling, from basic anatomy to the key small neurotransmitters, would expand our current understanding of neural-driven biological processes, such as growth and reproduction, and offers a new approach to exploring the propensity for COTS population explosions and subsequent collapse. In this study we investigated the metabolomic profiles of small molecule neurotransmitters in the COTS radial nerve cord. Multivariate analysis shows differential abundance of small molecule neurotransmitters in male and female COTS, and in food-deprived individuals with significant differences between sexes in gamma-aminobutyric acid (GABA), histamine and serotonin, and significant differences in histamine and serotonin between satiation states. Annotation established that the majority of biosynthesis enzyme genes are present in the COTS genome. The spatial distribution of GABA, histamine and serotonin in the radial nerve cord was subsequently confirmed by immunolocalization; serotonin is most prominent within the ectoneural regions, including unique neural bulbs, while GABA and histamine localize primarily within neuropil fibers. Glutamic acid, which was also found in high relative abundance and is a precursor of GABA, is known as a spawning inhibitor in seastars, and as such was tested for inhibition of ovulation ex-vivo which resulted in complete inhibition of oocyte maturation and ovulation induced by 1-Methyladenine. These findings not only advance our knowledge of echinoderm neural signaling processes but also identify potential targets for developing novel approaches for COTS biocontrol.
Related collections
Most cited references78
- Record: found
- Abstract: found
- Article: not found
A pragmatic and readily implemented quality control strategy for HPLC-MS and GC-MS-based metabonomic analysis.
- Record: found
- Abstract: found
- Article: not found