- Record: found
- Abstract: found
- Article: found
Exhaustion of Protective Heat Shock Response Induces Significant Tumor Damage by Apoptosis after Modulated Electro-Hyperthermia Treatment of Triple Negative Breast Cancer Isografts in Mice
Read this article at
Abstract
Simple Summary
Breast cancer is one of the most frequent cancer types among women worldwide. Triple-negative breast cancer is a highly aggressive breast cancer type with very poor survival due to the lack of targeted therapy. Modulated electro-hyperthermia (mEHT) is a newly emerging form of adjuvant, electromagnetic cancer-treatment. Capacitive energy delivery and frequency modulation enable the application of non-thermal effects. Furthermore, selective energy absorption by the tumor (as demonstrated in our present paper) enables 2.5 °C selective heating of the tumor. In the present study, we demonstrate in an in vivo syngeneic Balb/c TNBC mouse model that mEHT caused a remarkable reduction in the number of viable tumor cells accompanied by significant cleaved caspase-3-related apoptotic tumor tissue destruction and a transitional heat shock response. Furthermore, we demonstrated in vitro that the tumor cell killing effect of mEHT was amplified by inhibitors of the protective heat shock response such as Quercetin and KRIBB11.
Abstract
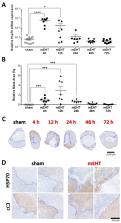
Modulated electro-hyperthermia (mEHT) is a complementary antitumor therapy applying capacitive radiofrequency at 13.56 MHz. Here we tested the efficiency of mEHT treatment in a BALB/c mouse isograft model using the firefly luciferase-transfected triple-negative breast cancer cell line, 4T1. Tumors inoculated orthotopically were treated twice using a novel ergonomic pole electrode and an improved mEHT device (LabEHY 200) at 0.7 ± 0.3 W for 30 min. Tumors were treated one, two, or three times every 48 h. Tumor growth was followed by IVIS, caliper, and ultrasound. Tumor destruction histology and molecular changes using immunohistochemistry and RT-qPCR were also revealed. In vivo, mEHT treatment transitionally elevated Hsp70 expression in surviving cells indicating heat shock-related cell stress, while IVIS fluorescence showed a significant reduction of viable tumor cell numbers. Treated tumor centers displayed significant microscopic tumor damage with prominent signs of apoptosis, and major upregulation of cleaved/activated caspase-3-positive tumor cells. Serial sampling demonstrated substantial elevation of heat shock (Hsp70) response twelve hours after the treatment which was exhausted by twenty-four hours after treatment. Heat shock inhibitors Quercetin or KRIBB11 could synergistically amplify mEHT-induced tumor apoptosis in vitro. In conclusion, modulated electro-hyperthermia exerted a protective heat shock response as a clear sign of tumor cell stress. Exhaustion of the HSR manifested in caspase-dependent apoptotic tumor cell death and tissue damage of triple-negative breast cancer after mEHT monotherapy. Inhibiting the HSR synergistically increased the effect of mEHT. This finding has great translational potential.
Related collections
Most cited references58
- Record: found
- Abstract: found
- Article: not found
Triple-negative breast cancer.
- Record: found
- Abstract: found
- Article: not found
The heat shock response: life on the verge of death.

- Record: found
- Abstract: found
- Article: found