- Record: found
- Abstract: found
- Article: found
Autophagy and Its Impact on Neurodegenerative Diseases: New Roles for TDP-43 and C9orf72
Read this article at
Abstract
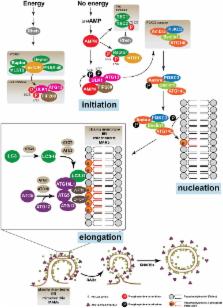
Autophagy is a catabolic mechanism where intracellular material is degraded by vesicular structures called autophagolysosomes. Autophagy is necessary to maintain the normal function of the central nervous system (CNS), avoiding the accumulation of misfolded and aggregated proteins. Consistently, impaired autophagy has been associated with the pathogenesis of various neurodegenerative diseases. The proteins TAR DNA-binding protein-43 (TDP-43), which regulates RNA processing at different levels, and chromosome 9 open reading frame 72 (C9orf72), probably involved in membrane trafficking, are crucial in the development of neurodegenerative diseases such as Amyotrophic lateral sclerosis (ALS) and Frontotemporal Lobar Degeneration (FTLD). Additionally, recent studies have identified a role for these proteins in the control of autophagy. In this manuscript, we review what is known regarding the autophagic mechanism and discuss the involvement of TDP-43 and C9orf72 in autophagy and their impact on neurodegenerative diseases.
Related collections
Most cited references107

- Record: found
- Abstract: found
- Article: found
Autophagosome formation from membrane compartments enriched in phosphatidylinositol 3-phosphate and dynamically connected to the endoplasmic reticulum
- Record: found
- Abstract: found
- Article: not found
Mitochondria supply membranes for autophagosome biogenesis during starvation.
- Record: found
- Abstract: found
- Article: not found