- Record: found
- Abstract: found
- Article: found
Cell Death Markers in Children with Immune Thrombocytopenic Purpura: A Preliminary Study
Read this article at
Abstract
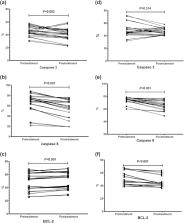
Immune thrombocytopenic purpura (ITP) is an autoimmune disease with possible dysregulation of the apoptotic pathways. We aimed to evaluate the possible role of some apoptotic markers (caspase 3, caspase 8 and BCL2) in the pathogenesis and course of ITP. We investigated some apoptotic markers (caspase 3, caspase 8 and BCL2) using the flow cytometry in 60 children with newly diagnosed ITP, 20 children with chemotherapy-related thrombocytopenia (CRT) and 20 healthy children. We also assessed the effects of intravenous immunoglobulin (IVIG) and methyl prednisolone therapies on the platelet apoptosis in children with newly diagnosed ITP. We demonstrated significantly higher values of caspase 3 in the newly diagnosed ITP group than control and CRT groups, and non-significantly higher values of caspase 8 in the ITP group than the healthy group. After IVIG treatment, the platelet count increased in all patients, and there was a significant decrease in caspase 3 and caspase 8 levels while BCL2 level increased. Regarding methylprednisolone treatment, there was a significant decrease in BCL2 and caspase 8 levels while caspase 3 levels did not significantly decrease. There is a possible role of the caspase dependent cell death pathway of the platelets in the occurrence of newly diagnosed ITP. There is heterogeneity in the apoptotic changes of newly diagnosed ITP children who received IVIG versus those who received methylprednisolone.
Related collections
Most cited references27
- Record: found
- Abstract: found
- Article: not found
The BCL-2 protein family: opposing activities that mediate cell death.
- Record: found
- Abstract: found
- Article: not found
Death and anti-death: tumour resistance to apoptosis.
- Record: found
- Abstract: found
- Article: not found