- Record: found
- Abstract: found
- Article: found
HNRNPA2B1-mediated m 6A modification of lncRNA MEG3 facilitates tumorigenesis and metastasis of non-small cell lung cancer by regulating miR-21-5p/PTEN axis
Read this article at
Abstract
Background
Accumulating data indicate that N6-methyladenosine (m 6A) RNA methylation and lncRNA deregulation act crucial roles in cancer progression. Heterogeneous nuclear ribonucleoprotein A2B1 (HNRNPA2B1) as an m 6A “reader” has been reported to be an oncogene in multiple malignancies. We herein aimed to elucidate the role and underlying mechanism by which HNRNPA2B1-mediated m 6A modification of lncRNAs contributes to non-small cell lung cancer (NSCLC).
Methods
The expression levels of HNRNPA2B1 and their association with the clinicopathological characteristics and prognosis in NSCLC were determined by RT-qPCR, Western blot, immunohistochemistry and TCGA dataset. Then, the role of HNRNPA2B1 in NSCLC cells was assessed by in vitro functional experiments and in vivo tumorigenesis and lung metastasis models. HNRNPA2B1-mediated m 6A modification of lncRNAs was screened by m 6A-lncRNA epi-transcriptomic microarray and verified by methylated RNA immunoprecipitation (Me-RIP). The lncRNA MEG3-specific binding with miR-21-5p was evaluated by luciferase gene report and RIP assays. The effects of HNRNPA2B1 and (or) lncRNA MEG3 on miR-21-5p/PTEN/PI3K/AKT signaling were examined by RT-qPCR and Western blot analyses.
Results
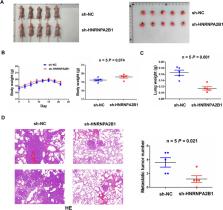
We found that upregulation of HNRNPA2B1 was associated with distant metastasis and poor survival, representing an independent prognostic factor in patients with NSCLC. Knockdown of HNRNPA2B1 impaired cell proliferation and metastasis in vitro and in vivo, whereas ectopic expression of HNRNPA2B1 possessed the opposite effects. Mechanical investigations revealed that lncRNA MEG3 was an m 6A target of HNRNPA2B1 and inhibition of HNRNPA2B1 decreased MEG3 m 6A levels but increased its mRNA levels. Furthermore, lncRNA MEG3 could act as a sponge of miR-21-5p to upregulate PTEN and inactivate PI3K/AKT signaling, leading to the suppression of cell proliferation and invasion. Low expression of lncRNA MEG3 or elevated expression of miR-21-5p indicated poor survival in patients with NSCLC.
Related collections
Most cited references46
- Record: found
- Abstract: found
- Article: not found
Cancer statistics, 2020
- Record: found
- Abstract: found
- Article: not found
m6A Modification in Coding and Non-coding RNAs: Roles and Therapeutic Implications in Cancer.

- Record: found
- Abstract: found
- Article: found