- Record: found
- Abstract: found
- Article: found
A single genetic locus controls both expression of DPEP1/CHMP1A and kidney disease development via ferroptosis
Read this article at
Abstract
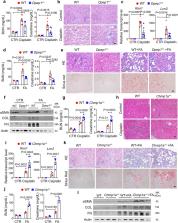
Genome-wide association studies (GWAS) have identified loci for kidney disease, but the causal variants, genes, and pathways remain unknown. Here we identify two kidney disease genes Dipeptidase 1 ( DPEP1) and Charged Multivesicular Body Protein 1 A ( CHMP1A) via the triangulation of kidney function GWAS, human kidney expression, and methylation quantitative trait loci. Using single-cell chromatin accessibility and genome editing, we fine map the region that controls the expression of both genes. Mouse genetic models demonstrate the causal roles of both genes in kidney disease. Cellular studies indicate that both Dpep1 and Chmp1a are important regulators of a single pathway, ferroptosis and lead to kidney disease development via altering cellular iron trafficking.
Abstract
Identifying causal variants and genes is an essential step in interpreting GWAS loci. Here, the authors investigate a kidney disease GWAS locus with functional genomics data, CRISPR editing and mouse experiments to identify DPEP1 and CHMP1A as putative kidney disease genes via ferroptosis.
Related collections
Most cited references55
- Record: found
- Abstract: found
- Article: not found
Ferroptosis: an iron-dependent form of nonapoptotic cell death.
- Record: found
- Abstract: found
- Article: not found
Ferroptosis: A Regulated Cell Death Nexus Linking Metabolism, Redox Biology, and Disease
- Record: found
- Abstract: found
- Article: not found