- Record: found
- Abstract: found
- Article: found
Molecular signatures distinguish senescent cells from inflammatory cells in aged mouse callus stromal cells
Read this article at
Abstract
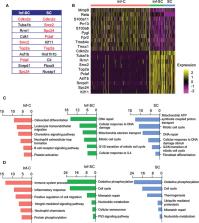
Cellular senescence plays important roles in age-related diseases, including musculoskeletal disorders. Senescent cells (SCs) exert a senescence-associated secretory phenotype (SASP) by producing SASP factors, some of which overlap with factors produced by inflammatory cells (Inf-Cs). However, the differences between SCs and Inf-Cs and how they interact with each other during fracture repair have not been well studied. Here, we analyzed single cell RNA sequencing data of aged mouse fracture callus stromal cells. We defined Inf-Cs as cells that express NF-κB Rela/Relb, SCs as cells that express the senescence genes, Cdkn1a, Cdkn2a or Cdkn2c, and inflammatory SCs (Inf-SCs) as cells that express both NF-κB and senescence genes. Differentially expressed genes and pathway analyses revealed that Inf-SCs and SCs had a similar gene expression profile and upregulated pathways that are related to DNA damage/oxidation-reduction and cellular senescence, while Inf-Cs expressed different gene signatures and pathways from SCs and Inf-SCs, mainly related to inflammation. Cellchat software analysis indicated that SCs and Inf-SCs are potential ligand-producing cells that affect Inf-Cs as target cells. Cell culture experiments demonstrated that SC conditioned medium promoted inflammatory gene expression by callus-derived mesenchymal progenitor cells, and Inf-Cs had reduced osteoblast differentiation capacity. In summary, we have identified three cell subclusters associated with inflammation and senescence in callus stromal cells, predicted potential effects of Inf-SCs and SCs on Inf-Cs by production of active ligands, and demonstrated that when mesenchymal progenitors acquire inflammatory phenotypes their osteogenic potential is reduced.
Related collections
Most cited references50
- Record: found
- Abstract: found
- Article: not found
Comprehensive Integration of Single-Cell Data

- Record: found
- Abstract: found
- Article: found
NF-κB signaling in inflammation

- Record: found
- Abstract: found
- Article: found