- Record: found
- Abstract: found
- Article: found
Serious infections in ANCA-associated vasculitides in the biologic era: real-life data from a multicenter cohort of 162 patients
Read this article at
Abstract
Background
Serious infections (SI) are common in patients with ANCA-associated vasculitides (AAV) like granulomatosis with polyangiitis (GPA) and microscopic polyangiitis (MPA). Real-life data regarding their incidence and predisposing factors—after the introduction of B cell depleting agents—are limited while data quantifying the risk per treatment modality and year of the disease are missing. Here, we aim to describe in details the incidence and the risk factors for SI in a contemporary AAV cohort.
Methods
Multicenter, observational, retrospective study of AAV patients followed in three tertiary referral centers.
Results
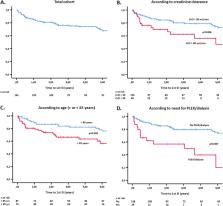
We included 162 patients with GPA (63%) and MPA (37%), males 51.9%, mean age 60.9 years, ΑΝCA+ 86%, and generalized disease 80%. During follow-up (891.2 patient-years, mean 5.4 years), 67 SI were recorded in 50 patients at an incidence rate of 7.5 per 100 patient-years. The SI incidence rate was higher during induction with cyclophosphamide (CYC) compared to rituximab (RTX, 19.3 vs. 11.3 per 100 patient-years, respectively) while it was lower and comparable between RTX and other regimens (5.52 vs. 4.54 per 100 patient-years, respectively) in the maintenance phase. By multivariate analysis, plasmapheresis (PLEX) and/or dialysis was a strong predictor for an SI during the 1st year after diagnosis (OR = 3.16, 95% CI 1.001–9.96) and throughout the follow-up period (OR = 5.21, 95% CI 1.93–14.07). In contrast, a higher baseline BVAS (OR = 1.11, 95% CI 1.01–1.21) was associated with SI only during the 1st year.
Conclusions
In this real-life study of patients with AAV, the SI incidence was higher during CYC compared to RTX induction while there was no difference between RTX and other agents used for maintenance therapy. Higher disease activity at baseline and need for PLEX and/or dialysis were independent factors associated with an SI.
Related collections
Most cited references21
- Record: found
- Abstract: found
- Article: not found
Rituximab versus cyclophosphamide for ANCA-associated vasculitis.
- Record: found
- Abstract: found
- Article: not found
EULAR/ERA-EDTA recommendations for the management of ANCA-associated vasculitis.
- Record: found
- Abstract: found
- Article: not found