- Record: found
- Abstract: found
- Article: not found
Distinct functionality of dishevelled isoforms on Ca 2+/calmodulin-dependent protein kinase 2 (CamKII) in Xenopus gastrulation
Read this article at
Abstract
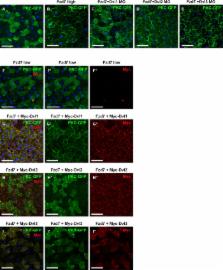
CamKII is a novel binding partner of Arrb2/Dvl2 protein complexes and is required for convergent extension movements in Xenopus. CamKII physically and functionally interacts with Dvl2, whereas CamKII activity is antagonistically modulated by Dvl1 and Dvl3.
Abstract
Wnt ligands trigger the activation of a variety of β-catenin–dependent and β-catenin–independent intracellular signaling cascades. Despite the variations in intracellular signaling, Wnt pathways share the effector proteins frizzled, dishevelled, and β-arrestin. It is unclear how the specific activation of individual branches and the integration of multiple signals are achieved. We hypothesized that the composition of dishevelled–β-arrestin protein complexes contributes to signal specificity and identified CamKII as an interaction partner of the dishevelled–β-arrestin protein complex by quantitative functional proteomics. Specifically, we found that CamKII isoforms interact differentially with the three vertebrate dishevelled proteins. Dvl1 is required for the activation of CamKII and PKC in the Wnt/Ca 2+ pathway. However, CamKII interacts with Dvl2 but not with Dvl1, and Dvl2 is necessary to mediate CamKII function downstream of Dvl1 in convergent extension movements in Xenopus gastrulation. Our findings indicate that the different Dvl proteins and the composition of dishevelled–β-arrestin protein complexes contribute to the specific activation of individual branches of Wnt signaling.
Related collections
Most cited references78
- Record: found
- Abstract: not found
- Article: not found
Wnt signaling: a common theme in animal development.
- Record: found
- Abstract: found
- Article: not found
Wnt induces LRP6 signalosomes and promotes dishevelled-dependent LRP6 phosphorylation.
- Record: found
- Abstract: found
- Article: not found
