- Record: found
- Abstract: found
- Article: found
Role of adaptin protein complexes in intracellular trafficking and their impact on diseases

Read this article at
ABSTRACT
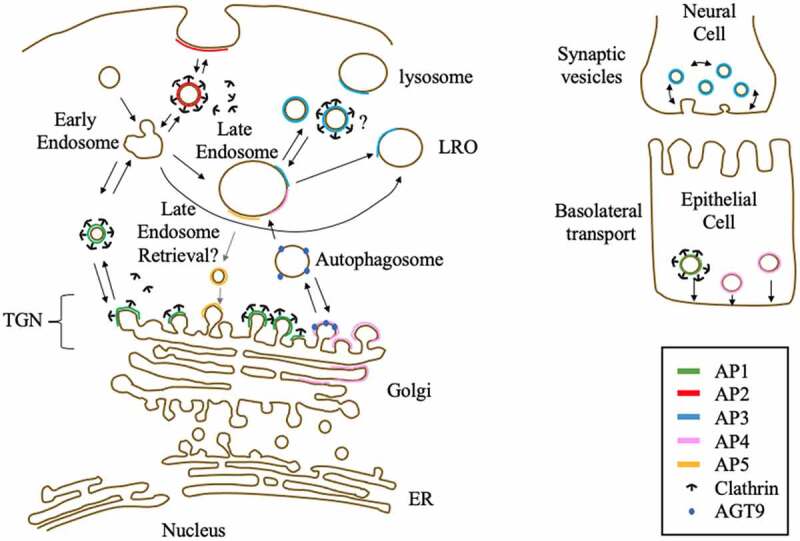
Adaptin proteins (APs) play a crucial role in intracellular cell trafficking. The ‘classical’ role of APs is carried out by AP1‒3, which bind to clathrin, cargo, and accessory proteins. Accordingly, AP1–3 are crucial for both vesicle formation and sorting. All APs consist of four subunits that are indispensable for their functions. In fact, based on studies using cells, model organism knockdown/knock-out, and human variants, each subunit plays crucial roles and contributes to the specificity of each AP. These studies also revealed that the sorting and intracellular trafficking function of AP can exert varying effects on pathology by controlling features such as cell development, signal transduction related to the apoptosis and proliferation pathways in cancer cells, organelle integrity, receptor presentation, and viral infection. Although the roles and functions of AP1‒3 are relatively well studied, the functions of the less abundant and more recently identified APs, AP4 and AP5, are still to be investigated. Further studies on these APs may enable a better understanding and targeting of specific diseases.
APs known or suggested locations and functions.
GRAPHICAL ABSTRACT
Related collections
Most cited references283
- Record: found
- Abstract: found
- Article: not found
Signals for sorting of transmembrane proteins to endosomes and lysosomes.
- Record: found
- Abstract: found
- Article: not found
Endocytic mechanisms for targeted drug delivery.
- Record: found
- Abstract: found
- Article: found