- Record: found
- Abstract: found
- Article: found
Synthesis, Spectroscopic and Theoretical Studies of New Quasi-Podands from Bile Acid Derivatives Linked by 1,2,3-Triazole Rings
research-article
24 February 2014
Read this article at
There is no author summary for this article yet. Authors can add summaries to their articles on ScienceOpen to make them more accessible to a non-specialist audience.
Abstract
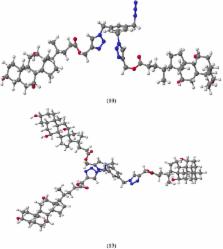
A novel method for the synthesis of bile acid derivatives has been developed using “click chemistry”. Intermolecular 1,3-dipolar cycloaddition of the propargyl ester of bile acids and azide groups of 1,3,5-tris(azidomethyl)benzene gave a new quasi-podands with 1,2,3-triazole rings. The structures of the products were confirmed by spectral ( 1H-NMR, 13C-NMR, and FT-IR) analysis, mass spectrometry and PM5 semiempirical methods. Estimation of the pharmacotherapeutic potential has been accomplished for synthesized compounds on the basis of Prediction of Activity Spectra for Substances (PASS).
Related collections
Most cited references44
- Record: found
- Abstract: not found
- Article: not found
Optimization of parameters for semiempirical methods I. Method
James J. P. Stewart (1989)
- Record: found
- Abstract: not found
- Article: not found
“On Water”: Unique Reactivity of Organic Compounds in Aqueous Suspension
Sridhar Narayan, John Muldoon, M Finn … (2005)
- Record: found
- Abstract: found
- Article: not found
Click chemistry reactions in medicinal chemistry: applications of the 1,3-dipolar cycloaddition between azides and alkynes.
Gian Cesare Tron, Tracey Pirali, Richard Billington … (2008)