- Record: found
- Abstract: found
- Article: found
Clinical Characteristics of Rapid Progression in Asia-Pacific Patients With ADPKD

Read this article at
Abstract
Introduction
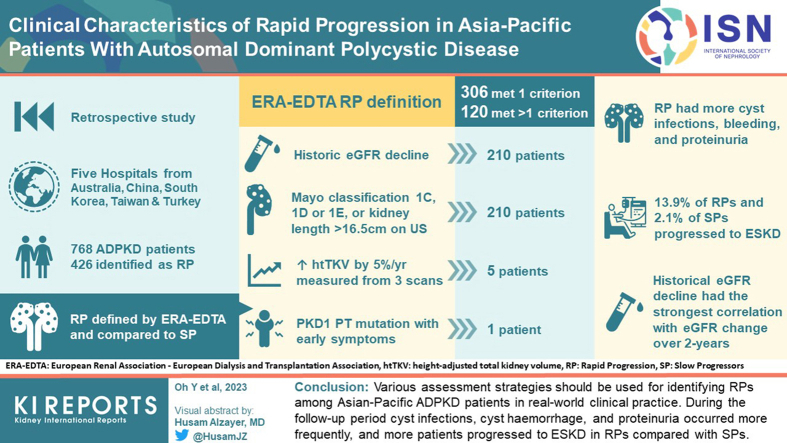
This study aimed to determine the utility of different methods to predict rapid progressors (RPs) and their clinical characteristics in Asia-Pacific patients with autosomal dominant polycystic kidney disease (ADPKD).
Methods
This was a multinational retrospective observational cohort study of patients with ADPKD in the Asia-Pacific region. Five hospitals from Australia, China, South Korea, Taiwan, and Turkey participated in this study. RP was defined by European Renal Association–European Dialysis and Transplantation Association (ERA-EDTA) guidelines and compared to slow progressors (SPs).
Results
Among 768 patients, 426 patients were RPs. Three hundred six patients met only 1 criterion and 120 patients satisfied multiple criteria for RP. Historical estimated glomerular filtration rate (eGFR) decline fulfilled the criteria for RP in 210 patients. Five patients met the criteria for a historical increase in height-adjusted total kidney volume (TKV). The 210 patients satisfied the criteria for based on kidney volume. During the follow-up period, cyst infections, cyst hemorrhage, and proteinuria occurred more frequently in RP; and 13.9% and 2.1% of RPs and SPs, respectively, progressed to end-stage kidney disease (ESKD). RP criteria based on historical eGFR decline had the strongest correlation with eGFR change over a 2-year follow-up
Conclusion
Various assessment strategies should be used for identifying RPs among Asian-Pacific patients with ADPKD in real-world clinical practice during the follow-up period, cyst infections, cyst hemorrhage, and proteinuria occurred more frequently; and more patients progressed to ESKD in RPs compared with SPs.
Graphical abstract
Related collections
Most cited references28
- Record: found
- Abstract: found
- Article: not found
A new equation to estimate glomerular filtration rate.
- Record: found
- Abstract: found
- Article: not found
Autosomal dominant polycystic kidney disease.
- Record: found
- Abstract: found
- Article: not found