- Record: found
- Abstract: found
- Article: found
Ozonolysis of 2-Methyl-2-pentenal: New Insights from Master Equation Modeling
Read this article at
Abstract

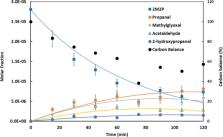
Experimental and theoretical studies were carried out to investigate the ozonolysis of trans-2-methyl-2-pentenal. The experiments were conducted in atmospheric simulation chambers coupled to a Fourier transform infrared (FTIR) spectrometer and a gas chromatograph–mass spectrometer at room temperature and atmospheric pressure in the presence of an excess of cyclohexane in dry conditions (RH < 1%). The ozonolysis reaction was investigated theoretically from the results of accurate density functional (M06-2X) and ab initio [CCSD(T)] computations, employing the AVTZ basis set. The sequence of reaction steps was established, and the system of kinetics equations was modeled using MESMER. In the first step, a primary ozonide is formed, which then decomposes along two pathways. The principal ozonolysis products are propanal, methylglyoxal, ethylformate, and a secondary ozonide. An interesting competition between sequential reaction steps and well-skipping is found, which leads to an inversion of the expected methylglyoxal/propanal product ratio at temperatures below 210 K. The mechanism of the “hot ester” reaction channel of the Criegee intermediate was revisited. The computed ozonolysis rate constant and product branching ratio are in excellent agreement with the experimental data that are also reported in the present work.
Related collections
Most cited references36
- Record: found
- Abstract: not found
- Article: not found
Atmospheric degradation of volatile organic compounds.
- Record: found
- Abstract: not found
- Article: not found