- Record: found
- Abstract: found
- Article: found
Development of a Kidney Prognostic Score in a Japanese Cohort of Patients With Antineutrophil Cytoplasmic Autoantibody Vasculitis

Read this article at
Abstract
Introduction
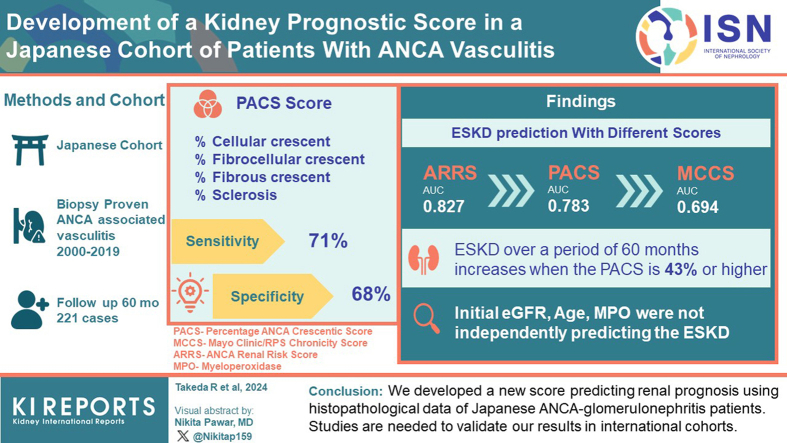
Glomerulonephritis is frequent in antineutrophil cytoplasmic antibody (ANCA)-associated vasculitis (AAV) and crucial to disease outcomes. We conducted a detailed assessment of renal pathology in Japanese patients with AAV, and developed a new score that would predict renal outcome.
Methods
Two hundred twenty-one patients who were diagnosed with AAV and underwent a kidney biopsy were enrolled. Data on glomerular, tubular, interstitial, and vascular lesions from kidney biopsies were analyzed; the 3 established classification and prognostic scoring systems (Berden Classification, Mayo Clinic/RPS Chronicity Score [MCCS], and ANCA Renal Risk Score [ARRS]) were validated. Further, we developed a new prognostic score by including variables relevant for Japanese patients with ANCA-glomerulonephritis.
Results
Median follow-up was 60 months (interquartile range: 6–60). End-stage kidney disease (ESKD) risk prediction by the MCCS and the ARRS was confirmed. Moreover, our analysis identified 4 items with significant ESKD risk prediction capacity, namely percentage of cellular, fibrocellular, and fibrous crescents; and sclerotic glomeruli. Based on our findings, we created a score evaluating the percentage of these lesions to total glomeruli, the Percentage of ANCA Crescentic Score (PACS). The area under the receiver operating characteristic (ROC) curve evaluating PACS was 0.783. The PACS had a comparable performance as the ARRS in predicting ESKD. The optimal PACS cut-off for ESKD risk over 60 months was 43%. In addition, the percentage of cellular crescents and presence of interstitial inflammation were independent predictors of kidney function recovery.
Graphical abstract
Related collections
Most cited references56
- Record: found
- Abstract: not found
- Article: not found
2012 revised International Chapel Hill Consensus Conference Nomenclature of Vasculitides.
- Record: found
- Abstract: found
- Article: not found
Revised equations for estimated GFR from serum creatinine in Japan.
- Record: found
- Abstract: found
- Article: not found