- Record: found
- Abstract: found
- Article: found
The Sedoheptulose Kinase CARKL Directs Macrophage Polarization through Control of Glucose Metabolism

Read this article at
Summary
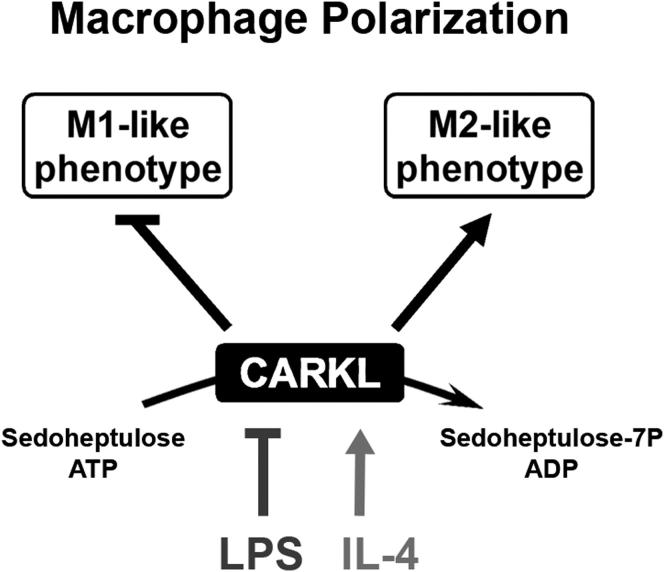
Immune cells are somewhat unique in that activation responses can alter quantitative phenotypes upwards of 100,000-fold. To date little is known about the metabolic adaptations necessary to mount such dramatic phenotypic shifts. Screening for novel regulators of macrophage activation, we found nonprotein kinases of glucose metabolism among the most enriched classes of candidate immune modulators. We find that one of these, the carbohydrate kinase- like protein CARKL, is rapidly downregulated in vitro and in vivo upon LPS stimulation in both mice and humans. Interestingly, CARKL catalyzes an orphan reaction in the pentose phosphate pathway, refocusing cellular metabolism to a high-redox state upon physiological or artificial downregulation. We find that CARKL-dependent metabolic reprogramming is required for proper M1- and M2-like macrophage polarization and uncover a rate-limiting requirement for appropriate glucose flux in macrophage polarization.
Abstract
Related collections
Most cited references36
- Record: found
- Abstract: found
- Article: not found
The inflammasomes: guardians of the body.
- Record: found
- Abstract: found
- Article: found