- Record: found
- Abstract: found
- Article: found
De novo design of picomolar SARS-CoV-2 miniprotein inhibitors
Read this article at
Miniproteins against SARS-CoV-2
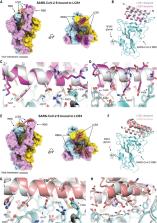
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is decorated with spikes, and viral entry into cells is initiated when these spikes bind to the host angiotensin-converting enzyme 2 (ACE2) receptor. Many monoclonal antibody therapies in development target the spike proteins. Cao et al. designed small, stable proteins that bind tightly to the spike and block it from binding to ACE2. The best designs bind with very high affinity and prevent SARS-CoV-2 infection of mammalian Vero E6 cells. Cryo–electron microscopy shows that the structures of the two most potent inhibitors are nearly identical to the computational models. Unlike antibodies, the miniproteins do not require expression in mammalian cells, and their small size and high stability may allow formulation for direct delivery to the nasal or respiratory system.
Science, this issue p. 426
Abstract
Designed miniproteins bind tightly to the SARS-CoV-2 spike protein and prevent binding to the host cell receptor.
Abstract
Targeting the interaction between the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) spike protein and the human angiotensin-converting enzyme 2 (ACE2) receptor is a promising therapeutic strategy. We designed inhibitors using two de novo design approaches. Computer-generated scaffolds were either built around an ACE2 helix that interacts with the spike receptor binding domain (RBD) or docked against the RBD to identify new binding modes, and their amino acid sequences were designed to optimize target binding, folding, and stability. Ten designs bound the RBD, with affinities ranging from 100 picomolar to 10 nanomolar, and blocked SARS-CoV-2 infection of Vero E6 cells with median inhibitory concentration (IC 50) values between 24 picomolar and 35 nanomolar. The most potent, with new binding modes, are 56- and 64-residue proteins (IC 50 ~ 0.16 nanograms per milliliter). Cryo–electron microscopy structures of these minibinders in complex with the SARS-CoV-2 spike ectodomain trimer with all three RBDs bound are nearly identical to the computational models. These hyperstable minibinders provide starting points for SARS-CoV-2 therapeutics.
Related collections
Most cited references38
- Record: found
- Abstract: found
- Article: not found
UCSF Chimera--a visualization system for exploratory research and analysis.
- Record: found
- Abstract: found
- Article: not found
Structure of the SARS-CoV-2 spike receptor-binding domain bound to the ACE2 receptor
- Record: found
- Abstract: found
- Article: not found