- Record: found
- Abstract: found
- Article: found
Excretory/Secretory Products From Trichinella spiralis Adult Worms Attenuated DSS-Induced Colitis in Mice by Driving PD-1-Mediated M2 Macrophage Polarization
Read this article at
Abstract
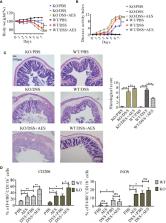
Helminth-modulated macrophages contribute to attenuating inflammation in inflammatory bowel diseases. The programmed death 1 (PD-1) plays an important role in macrophage polarization and is essential in the maintenance of immune system homeostasis. Here, we investigate the role of PD-1-mediated polarization of M2 macrophages and the protective effects of excretory/secretory products from Trichinella spiralis adult worms (AES) on DSS-induced colitis in mice. Colitis in mice was induced by oral administration of dextran sodium sulfate (DSS) daily. Mice with DSS-induced colitis were treated with T. spiralis AES intraperitoneally, and pathological manifestations were evaluated. Macrophages in mice were depleted with liposomal clodronate. Markers for M1-type (iNOS, TNF-α) and M2-type (CD206, Arg-1) macrophages were detected by qRT-PCR and flow cytometry. Macrophage expression of PD-1 was quantified by flow cytometry; RAW 264.7 cells and peritoneal macrophages were used for in vitro tests, and PD-1 gene knockout mice were used for in vivo investigation of the role of PD-1 in AES-induced M2 macrophage polarization. Macrophage depletion was found to reduce DSS-induced colitis in mice. Treatment with T. spiralis AES significantly increased macrophage expression of CD206 and Arg-1 and simultaneously attenuated colitis severity. We found T. spiralis AES to enhance M2 macrophage polarization; these findings were confirmed studying in vitro cultures of RAW264.7 cells and peritoneal macrophages from mice. Further experimentation revealed that AES upregulated PD-1 expression, primarily on M2 macrophages expressing CD206. The AES-induced M2 polarization was found to be decreased in PD-1 deficient macrophages, and the therapeutic effects of AES on colitis was reduced in PD-1 knockout mice. In conclusion, the protective effects of T. spiralis AES on DSS-induced colitis were found to associate with PD-1 upregulation and M2 macrophage polarization. Thus, PD-1-mediated M2 macrophage polarization is a key mechanism of helminth-induced modulation of the host immune system.
Related collections
Most cited references50
- Record: found
- Abstract: found
- Article: not found
PD-1 expression by tumor-associated macrophages inhibits phagocytosis and tumor immunity
- Record: found
- Abstract: not found
- Article: not found