- Record: found
- Abstract: found
- Article: found
Stereotactic Ablative Radiation Therapy in 3 Fractions Induces a Favorable Systemic Immune Cell Profiling in Prostate Cancer Patients
Read this article at
ABSTRACT
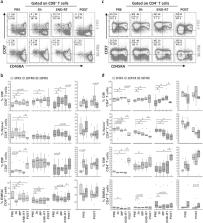
The impact of radiotherapy (RT) on immune cell status in prostate cancer (PCa) is only partially determined. The aim of this study was to assess the effect of different RT strategies on peripheral B, T, and Natural killer (NK) lymphocytes at precise longitudinal time-points in PCa. 18 patients treated with stereotactic body radiation therapy (SBRT) (40 Gy/3FRX), definitive moderate-hypofractionation (62 Gy/20FRX), or post-operative conventional-fractionation RT (66–69 Gy/30FRX) were prospectively evaluated for the immune cell profile in terms of immune cell composition, differentiation stage, cytokine production and inhibitory receptor (IR) expression. The immune-monitoring of the 18 patients revealed that RT affects the balance of systemic immune cells, with the main differences observed between SBRT and conventionally fractionated RT. SBRT favorably impacts immune response in term of increased B cells, central-memory and effector-memory CD8 + T cells, along with decreased Treg cells after treatment. On the contrary, conventional fractionated RT had a long-term negative effect on the systemic immune profile, including a decrease of total lymphocyte counts accompanied by an increase of neutrophils-to-lymphocytes ratio. Total B and T cells decreased and Treg-to-CD8 + ratio increased. Functionality of T lymphocytes were not affected by any of the 3-fractionation schedules. Interestingly, SBRT significantly up-regulates the expression of V-domain immunoglobulin suppressor of T-cell activation (VISTA) in CD8 + T cells in the absence of other IRs. Our results indicate the relevance of systematic immunomonitoring during RT to identify novel immune-related target to design trials of combined radio-immunotherapy as a promising strategy in the clinical management of PCa.
Related collections
Most cited references43
- Record: found
- Abstract: found
- Article: not found
Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries
- Record: found
- Abstract: found
- Article: not found
Ultra-hypofractionated versus conventionally fractionated radiotherapy for prostate cancer: 5-year outcomes of the HYPO-RT-PC randomised, non-inferiority, phase 3 trial

- Record: found
- Abstract: found
- Article: found