- Record: found
- Abstract: found
- Article: found
Transforming growth factor-β in stem cells and tissue homeostasis
Read this article at
Abstract
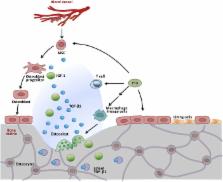
TGF-β 1–3 are unique multi-functional growth factors that are only expressed in mammals, and mainly secreted and stored as a latent complex in the extracellular matrix (ECM). The biological functions of TGF-β in adults can only be delivered after ligand activation, mostly in response to environmental perturbations. Although involved in multiple biological and pathological processes of the human body, the exact roles of TGF-β in maintaining stem cells and tissue homeostasis have not been well-documented until recent advances, which delineate their functions in a given context. Our recent findings, along with data reported by others, have clearly shown that temporal and spatial activation of TGF-β is involved in the recruitment of stem/progenitor cell participation in tissue regeneration/remodeling process, whereas sustained abnormalities in TGF-β ligand activation, regardless of genetic or environmental origin, will inevitably disrupt the normal physiology and lead to pathobiology of major diseases. Modulation of TGF-β signaling with different approaches has proven effective pre-clinically in the treatment of multiple pathologies such as sclerosis/fibrosis, tumor metastasis, osteoarthritis, and immune disorders. Thus, further elucidation of the mechanisms by which TGF-β is activated in different tissues/organs and how targeted cells respond in a context-dependent way can likely be translated with clinical benefits in the management of a broad range of diseases with the involvement of TGF-β.
Growth factor: Activation in health and disease
Targeting a critical growth factor involved in bone and other tissue remodeling could help treat osteoarthritis and other skeletal disorders. A team led by Zhou Xuedong from Sichuan University in Chengdu, China, and Xu Cao from the Johns Hopkins University School of Medicine in Baltimore, Maryland, USA, review the ways in which temporal and spatial activation of transforming growth factor-β (TGF-β), a multi-functional signaling molecule, are needed for proper tissue development and regulation of stem cells throughout the body. Looking at the skeletal system in particular, the researchers discuss how TGF-β controls the balance between bone resorption and bone formation. Faulty TGF-β signaling can lead to numerous bone-associated disorders, including rare genetic diseases and metastatic cancers. The authors also summarize clinical efforts to modulate TGF-β with drugs for the treatment of osteoarthritis and other conditions.
Related collections
Most cited references491
- Record: found
- Abstract: found
- Article: not found
Self-renewing osteoprogenitors in bone marrow sinusoids can organize a hematopoietic microenvironment.
- Record: found
- Abstract: found
- Article: not found