- Record: found
- Abstract: found
- Article: found
Assessment of aneuploidy formation in human blastocysts resulting from cryopreserved donor eggs
Read this article at
Abstract
Background
Increased embryo implantation rates were reported after transfer of euploid embryos selected by preimplantation genetic screening (PGS). Egg cryopreservation by vitrification has become one of the most important assisted human reproduction technologies. Although reports indicate that development and implantation of human embryos derived from frozen donor eggs are comparative to fresh eggs, it is still unknown whether egg vitrification increases chromosomal abnormalities in eggs, which in turn causes formation of embryonic aneuploidy. Therefore, in this study, we evaluated the aneuploidy formation in the blastocysts derived from frozen donor eggs and also evaluated the efficiency of egg vitrification as an advanced technology for egg cryopreservation.
Results
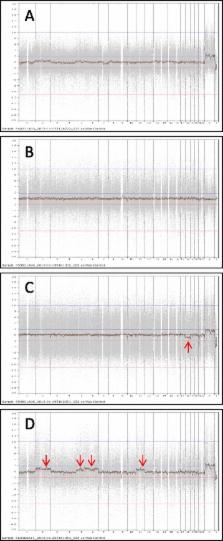
In this study, donated human eggs from young women were cryopreserved by vitrification and PGS was performed in the resulted blastocysts by DNA microarray. A total of 764 frozen eggs from 75 egg thawing cycles were warmed and 38 blastocysts were biopsied for PGS before embryo transfer. A 97.1% of egg survival rate was obtained and 59.1% of embryos developed to blastocyst stage. After biopsy and PGS, it was found that 84.2% of blastocysts were euploid and 15.8% were aneuploid. Aneuploidy rates varied among donors. Transfers of blastocysts without PGS resulted in higher clinical pregnancy and implantation rates as compared with transfer of blastocysts with PGS.
Conclusions
Although the overall aneuploidy rate was low in the blastocysts derived from frozen donor eggs, high aneuploidy rates were observed in the embryos resulting from some donated eggs. Clinical pregnancy rate was not improved by PGS of embryos resulting from donor eggs, indicating that PGS may not be necessary for embryos derived from donor eggs in most cases.
Related collections
Most cited references40
- Record: found
- Abstract: found
- Article: not found
Milder ovarian stimulation for in-vitro fertilization reduces aneuploidy in the human preimplantation embryo: a randomized controlled trial.
- Record: found
- Abstract: found
- Article: found
Embryo development of fresh ‘versus’ vitrified metaphase II oocytes after ICSI: a prospective randomized sibling-oocyte study
- Record: found
- Abstract: found
- Article: not found