- Record: found
- Abstract: found
- Article: found
Gut dysbiosis contributes to amyloid pathology, associated with C/EBPβ/AEP signaling activation in Alzheimer’s disease mouse model
Read this article at
Abstract
This study reveals the contribution of gut bacteria to Alzheimer’s disease.
Abstract
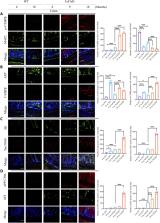
The gut-brain axis is bidirectional, and gut microbiota influence brain disorders including Alzheimer’s disease (AD). CCAAT/enhancer binding protein β/asparagine endopeptidase (C/EBPβ/AEP) signaling spatiotemporally mediates AD pathologies in the brain via cleaving both β-amyloid precursor protein and Tau. We show that gut dysbiosis occurs in 5xFAD mice, and is associated with escalation of the C/EBPβ/AEP pathway in the gut with age. Unlike that of aged wild-type mice, the microbiota of aged 3xTg mice accelerate AD pathology in young 3xTg mice, accompanied by active C/EBPβ/AEP signaling in the brain. Antibiotic treatment diminishes this signaling and attenuates amyloidogenic processes in 5xFAD, improving cognitive functions. The prebiotic R13 inhibits this pathway and suppresses amyloid aggregates in the gut. R13-induced Lactobacillus salivarius antagonizes the C/EBPβ/AEP axis, mitigating gut leakage and oxidative stress. Our findings support the hypothesis that C/EBPβ/AEP signaling is activated by gut dysbiosis, implicated in AD pathologies in the gut.
Related collections
Most cited references25
- Record: found
- Abstract: found
- Article: not found
Transfer of a healthy microbiota reduces amyloid and tau pathology in an Alzheimer’s disease animal model

- Record: found
- Abstract: found
- Article: found