- Record: found
- Abstract: found
- Article: found
Xiaoyankangjun tablet alleviates dextran sulfate sodium-induced colitis in mice by regulating gut microbiota and JAK2/STAT3 pathway
Read this article at
Abstract
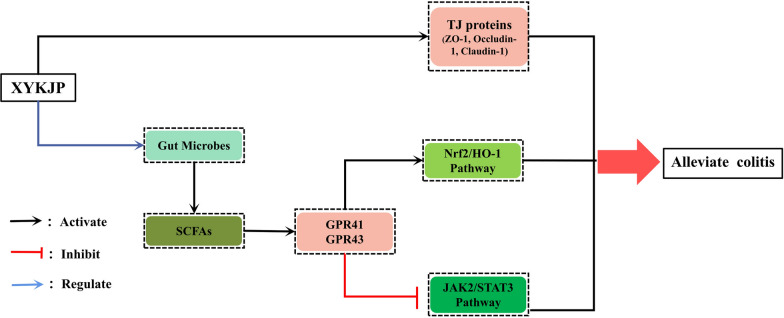
Xiaoyankangjun tablet (XYKJP) is a traditional Chinese medicine formulation used to treat intestinal disorders in clinical practice. However, the specific therapeutic mechanism of action of XYKJP in colitis has not yet been elucidated. This study aimed to reveal the multifaceted mechanisms of action of XYKJP in treating colitis. The model established based on DSS-induced colitis in C57BL/6 mice was employed to estimate the effect of XYKJP on colitis, which was then followed by histological assessment, 16S rRNA sequencing, RT-qPCR, ELISA, and Western blot. XYKJP alleviated the symptoms of DSS-induced colitis mainly by reducing oxidative stress, inflammatory responses, and intestinal mucosal repair in colitis tissues. In addition, XYKJP regulated the intestinal flora by increasing the relative abundance of Akkermansia and Bifidobacterium and reducing the relative abundance of Coriobacteriaceae_UCG-002. Mechanistically, XYKJP increased the content of short-chain fatty acids (SCFAs) in the feces, particularly propanoic acid and butyric acid, activated their specific receptor GPR43/41, furthermore activated the Nrf2/HO-1 pathway, and suppressed the JAK2/STAT3 pathway. XYKJP significantly alleviated the symptoms of experimental colitis and functioned synergistically by regulating the intestinal flora, increasing the production of SCFAs, and activating their specific receptors, thereby repressing oxidative stress and inflammation.
Related collections
Most cited references37
- Record: found
- Abstract: found
- Article: not found
Gut microbiota-derived metabolites as key actors in inflammatory bowel disease
- Record: found
- Abstract: found
- Article: not found
Pathway paradigms revealed from the genetics of inflammatory bowel disease
- Record: found
- Abstract: not found
- Article: not found