- Record: found
- Abstract: found
- Article: found
Detection of Replication Competent Lentivirus Using a qPCR Assay for VSV-G
Read this article at
Abstract
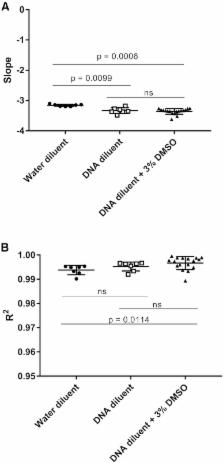
Lentiviral vectors are a common tool used to introduce new and corrected genes into cell therapy products for treatment of human diseases. Although lentiviral vectors are ideal for delivery and stable integration of genes of interest into the host cell genome, they potentially pose risks to human health, such as integration-mediated transformation and generation of a replication competent lentivirus (RCL) capable of infecting non-target cells. In consideration of the latter risk, all cell-based products modified by lentiviral vectors and intended for patient use must be tested for RCL prior to treatment of the patient. Current Food and Drug Administration (FDA) guidelines recommend use of cell-based assays to this end, which can take up to 6 weeks for results. However, qPCR-based assays are a quick alternative for rapid assessment of RCL in products intended for fresh infusion. We describe here the development and qualification of a qPCR assay based on detection of envelope gene sequences (vesicular stomatitis virus G glycoprotein [ VSV-G]) for RCL in accordance with Minimum Information for Publication of Quantitative Real-Time PCR Experiments (MIQE) guidelines. Our results demonstrate the sensitivity, linearity, specificity, and reproducibility of detection of VSV-G sequences, with a low false-positive rate. These procedures are currently being used in our phase 1 clinical investigations.
Related collections
Most cited references17
- Record: found
- Abstract: found
- Article: not found
In vivo gene delivery and stable transduction of nondividing cells by a lentiviral vector.
- Record: found
- Abstract: found
- Article: not found
Self-inactivating lentivirus vector for safe and efficient in vivo gene delivery.
- Record: found
- Abstract: found
- Article: not found