- Record: found
- Abstract: found
- Article: found
Valosin-containing protein (VCP) is required for autophagy and is disrupted in VCP disease
Read this article at
Abstract
Accumulation of autophagosomes because of impaired autophagy during valosin-containing protein (VCP)–linked dementia is explained by the absence or reduced activity of VCP.
Abstract
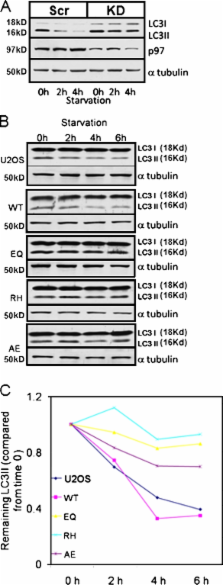
Mutations in valosin-containing protein (VCP) cause inclusion body myopathy (IBM), Paget's disease of the bone, and frontotemporal dementia (IBMPFD). Patient muscle has degenerating fibers, rimmed vacuoles (RVs), and sarcoplasmic inclusions containing ubiquitin and TDP-43 (TARDNA-binding protein 43). In this study, we find that IBMPFD muscle also accumulates autophagosome-associated proteins, Map1-LC3 (LC3), and p62/sequestosome, which localize to RVs. To test whether VCP participates in autophagy, we silenced VCP or expressed adenosine triphosphatase–inactive VCP. Under basal conditions, loss of VCP activity results in autophagosome accumulation. After autophagic induction, these autophagosomes fail to mature into autolysosomes and degrade LC3. Similarly, IBMPFD mutant VCP expression in cells and animals leads to the accumulation of nondegradative autophagosomes that coalesce at RVs and fail to degrade aggregated proteins. Interestingly, TDP-43 accumulates in the cytosol upon autophagic inhibition, similar to that seen after IBMPFD mutant expression. These data implicate VCP in autophagy and suggest that impaired autophagy explains the pathology seen in IBMPFD muscle, including TDP-43 accumulation.
Related collections
Most cited references36
- Record: found
- Abstract: found
- Article: not found
Autophagy Inhibition Compromises Degradation of Ubiquitin-Proteasome Pathway Substrates
- Record: found
- Abstract: found
- Article: not found