- Record: found
- Abstract: found
- Article: found
Management of Non-response and Loss of Response to Anti-tumor Necrosis Factor Therapy in Inflammatory Bowel Disease

Read this article at
Abstract
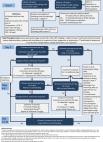
Anti-tumor necrosis factor (anti-TNF) therapy has been successfully used as first-line biologic treatment for moderate-to-severe inflammatory bowel disease (IBD), in both “step-up” and “top-down” approaches, and has become a cornerstone of IBD management. However, in a proportion of patients the effectiveness of anti-TNF therapy is sub-optimal. Either patients do not achieve adequate initial response (primary non-response) or they lose response after initial success (loss of response). Therapeutic drug monitoring determines drug serum concentrations and the presence of anti-drug antibodies (ADAbs) and can help guide treatment optimization to improve patient outcomes. For patients with low drug concentrations who are ADAb-negative or display low levels of ADAbs, dose escalation is recommended. Should response remain unchanged following dose optimization the question whether to switch within class (anti-TNF) or out of class (different mechanism of action) arises. If ADAb levels are high and the patient has previously benefited from anti-TNF therapy, then switching within class is a viable option as ADAbs are molecule specific. Addition of an immunomodulator may lead to a decrease in ADAbs and a regaining of response in a proportion of patients. If a patient does not achieve a robust therapeutic response with an initial anti-TNF despite adequate drug levels, then switching out of class is appropriate. In conjunction with the guidance above, other factors including patient preference, age, comorbidities, disease phenotype, extra-intestinal manifestations, and treatment costs need to be factored into the treatment decision. In this review we discuss current evidence in this field and provide guidance on therapeutic decision-making in clinical situations.
Related collections
Most cited references173
- Record: found
- Abstract: found
- Article: not found
Infliximab for induction and maintenance therapy for ulcerative colitis.
- Record: found
- Abstract: found
- Article: not found
STRIDE-II: An Update on the Selecting Therapeutic Targets in Inflammatory Bowel Disease (STRIDE) Initiative of the International Organization for the Study of IBD (IOIBD): Determining Therapeutic Goals for Treat-to-Target strategies in IBD
- Record: found
- Abstract: found
- Article: found
British Society of Gastroenterology consensus guidelines on the management of inflammatory bowel disease in adults
Author and article information
Comments
Comment on this article
 Smart Citations
Smart CitationsSee how this article has been cited at scite.ai
scite shows how a scientific paper has been cited by providing the context of the citation, a classification describing whether it supports, mentions, or contrasts the cited claim, and a label indicating in which section the citation was made.