- Record: found
- Abstract: found
- Article: not found
Therapeutic silencing of miR-10b inhibits metastasis in a mouse mammary tumor model
Read this article at
Abstract
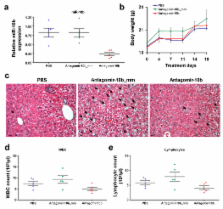
MicroRNAs (miRNAs) are increasingly implicated in regulating metastasis. Despite progress in silencing miRNAs in normal tissues of rodents and non-human primates, the development of effective approaches for sequence-specific inhibition of miRNAs in fast-growing tumors remains a significant scientific and clinical challenge. Here we show that systemic treatment of tumor-bearing mice with miR-10b antagomirs – a class of chemically modified anti-miRNA oligonucleotides – suppresses breast cancer metastasis. Silencing of miR-10b both in vitro and in vivo with antagomirs significantly decreases miR-10b levels and increases levels of a functionally important miR-10b target, Hoxd10. Administration of miR-10b antagomirs to mice bearing highly metastatic cells does not reduce primary mammary tumor growth but instead markedly suppresses formation of lung metastases. This metastasis-suppressing effect is sequence-specific. The miR-10b antagomir, which is well tolerated by normal animals, appears to be a promising candidate and a starting point for the development of new anti-metastasis agents.
Related collections
Most cited references22
- Record: found
- Abstract: found
- Article: not found
A randomized trial of bevacizumab, an anti-vascular endothelial growth factor antibody, for metastatic renal cancer.
- Record: found
- Abstract: found
- Article: not found
Selective events in the metastatic process defined by analysis of the sequential dissemination of subpopulations of a mouse mammary tumor.
- Record: found
- Abstract: found
- Article: not found
