- Record: found
- Abstract: found
- Article: found
Role of microRNAs in programmed cell death in renal diseases: A review
Read this article at
Abstract
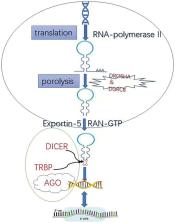
MicroRNAs (miRNAs) regulate gene expression involving kidney morphogenesis and cell proliferation, apoptosis, differentiation, migration, invasion, immune evasion, and extracellular matrix remodeling. Programmed cell death (PCD) is mediated and regulated by specific genes and a wealth of miRNAs, which participate in various pathological processes. Dysregulation of miRNAs can disrupt renal development and induce the onset and progression of various renal diseases. An in-depth understanding of how miRNAs regulate renal development and diseases is indispensable to comprehending how they can be used in new diagnostic and therapeutic approaches. However, the mechanisms are still insufficiently investigated. Hence, we review the current roles of miRNA-related signaling pathways and recent advances in PCD research and aim to display the potential crosstalk between miRNAs and PCD. The prospects of miRNAs as novel biomarkers and therapeutic targets are also described, which might provide some novel ideas for further studies.
Related collections
Most cited references92

- Record: found
- Abstract: found
- Article: found
Overview of MicroRNA Biogenesis, Mechanisms of Actions, and Circulation
- Record: found
- Abstract: found
- Article: not found
MicroRNA therapeutics: towards a new era for the management of cancer and other diseases
