- Record: found
- Abstract: found
- Article: found
Dysregulation of iron homeostasis and ferroptosis in sevoflurane and isoflurane associated perioperative neurocognitive disorders
Read this article at
Abstract
In recent years, sevoflurane and isoflurane are the most popular anesthetics in general anesthesia for their safe, rapid onset, and well tolerant. Nevertheless, many studies reported their neurotoxicity among pediatric and aged populations. This effect is usually manifested as cognitive impairment such as perioperative neurocognitive disorders. The wide application of sevoflurane and isoflurane during general anesthesia makes their safety a major health concern. Evidence indicates that iron dyshomeostasis and ferroptosis may establish a role in neurotoxicity of sevoflurane and isoflurane. However, the mechanisms of sevoflurane‐ and isoflurane‐induced neuronal injury were not fully understood, which poses a barrier to the treatment of its neurotoxicity. We, therefore, reviewed the current knowledge on mechanisms of iron dyshomeostasis and ferroptosis and aimed to promote a better understanding of their roles in sevoflurane‐ and isoflurane‐induced neurotoxicity.
Abstract
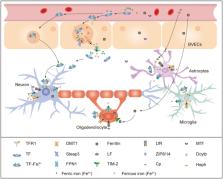
Current knowledge on sevoflurane and isoflurane's effects on iron metabolism and ferroptosis in vulnerable brain. Sevoflurane and isoflurane anesthesia interrupt iron homeostasis and result in ferroptosis in brain. The expression of iron transporters including TFR1, FPN1, and DMT1 is impacted by sevoflurane anesthesia. Sevoflurane anesthesia causes iron overload via NMDAR‐RASD1‐DMT1 pathway‐mediated iron uptake. Iron overload induced by sevoflurane treatment can lead to Aβ accumulation, mitochondrial dysfunction, lipid ROS accumulation, and cognitive impairment in developmental or aging rodents. DFP and Fer‐1 can reverse these negative changes. SLC7A11‐GSH‐GPX4 axis is implicated into sevoflurane and isoflurane induced ferroptosis. Factors regulating this crucial axis are p‐Becline, MIB2, and ATF4. Echinatin inhibits sevoflurane‐induced ferroptosis by upregulatintg NRF2 pathway. In addition, mitochondria protective agents elamipretide (SS‐31), DMF and Mito‐Tempo treatment improved sevoflurane‐ and isoflurane‐induced cognitive deficits.
Related collections
Most cited references112
- Record: found
- Abstract: found
- Article: not found
Ferroptosis: an iron-dependent form of nonapoptotic cell death.
- Record: found
- Abstract: found
- Article: not found
Ferroptosis: A Regulated Cell Death Nexus Linking Metabolism, Redox Biology, and Disease

- Record: found
- Abstract: found
- Article: found
