- Record: found
- Abstract: found
- Article: found
SORBS1 inhibits epithelial to mesenchymal transition (EMT) of breast cancer cells by regulating PI3K/AKT signaling and macrophage phenotypic polarization
Read this article at
Abstract
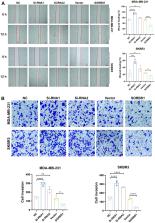
This study aimed to explore the regulatory role of SORBS1 in macrophage polarization and the PI3K/AKT signaling pathway, as well as analyze its mechanism in epithelial-mesenchymal transition (EMT) of breast cancer cells. We established SORBS1-overexpressing and knockout cell lines and verified the effects of SORBS1 on cell viability, invasion, and migration by phenotyping experiments and assaying the expression of associated proteins. Furthermore, we established a breast cancer cell and macrophage co-culture system to validate the effect of SORBS1 expression on macrophage polarization and killing of breast cancer cells. Bioinformatics analysis showed that SORBS1 was lowly expressed in breast cancer (BRCA) samples and highly expressed in healthy tissues. Decreased SORBS1 expression was associated with poor prognosis, and the PI3K/AKT signaling pathway was the most significantly enriched pathway. In vitro experiments showed that high expression of SORBS1 inhibited the migration of breast cancer cells, as well as the PI3K/AKT signaling pathway, and blocked EMT of these cells. In addition, SORBS1 induced macrophage polarization to the M1-type and enhanced the killing effect on breast cancer cells in the co-culture system. In conclusion, we successfully verified that SORBS1 inhibits the invasion and migration of breast cancer cells, induces macrophage M1-type polarization, and blocks EMT of breast cancer cells, and it may act by regulating the PI3K/AKT signaling pathway.
Related collections
Most cited references28

- Record: found
- Abstract: found
- Article: found
UALCAN: A Portal for Facilitating Tumor Subgroup Gene Expression and Survival Analyses1
- Record: found
- Abstract: found
- Article: found