- Record: found
- Abstract: found
- Article: found
Significance of CD103 + tissue-resident memory T cells for predicting the effectiveness of immune checkpoint inhibitors in esophageal cancer
Read this article at
Abstract
Background
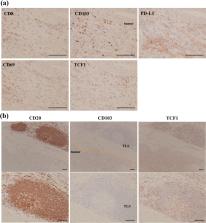
Immune checkpoint inhibitors (ICIs), including nivolumab, have been approved to treat esophageal cancer. However, these remedies are not fit for all patients with esophageal cancer; therefore, a predictive surrogate marker is needed to assess their effectiveness. CD103 +CD8 + tumor-infiltrating lymphocytes, defined as tissue-resident memory T cells (T RM), are promising indicators of response to ICIs, but it remains to be elucidated. This study investigated the association between the efficacy of ICIs and T RM.
Methods
The relationships between T RM infiltrating esophageal cancer, clinicopathological features, and prognosis after nivolumab initiation were examined using immunostaining. Tissue samples were obtained from surgically resected specimens of 37 patients with esophageal cancer who received nivolumab as a secondary or subsequent therapy. In addition, T RM infiltration was compared with programmed death-ligand 1 (PD-L1) expression and blood count parameters as predictors of nivolumab effectiveness.
Results
T RM-rich patients had a significant survival benefit after nivolumab initiation (12-months overall survival 70.8% vs 37.2%, p = 0.0485; 12-months progression-free survival 31.2% vs 0%, p = 0.0153) and experienced immune-related adverse events more frequently than T RM-poor patients (6 vs 2 patients). T RM infiltration was weakly correlated with PD-L1 positivity ( r = 0.374, p = 0.022), but T RM may indicate more sensitive response to ICIs than PD-L1 expression in this study. Some blood test parameters also weakly correlated with T RM but did not impact prognosis.
Related collections
Most cited references40
- Record: found
- Abstract: found
- Article: not found
Nivolumab versus Everolimus in Advanced Renal-Cell Carcinoma.
- Record: found
- Abstract: found
- Article: not found
B cells and tertiary lymphoid structures promote immunotherapy response
- Record: found
- Abstract: found
- Article: not found