- Record: found
- Abstract: found
- Article: not found
Effects of Stimulants on Brain Function in Attention-Deficit/Hyperactivity Disorder: A Systematic Review and Meta-Analysis
Read this article at
Abstract
Background
Psychostimulant medication, most commonly the catecholamine agonist methylphenidate, is the most effective treatment for attention-deficit/hyperactivity disorder (ADHD). However, relatively little is known on the mechanisms of action. Acute effects on brain function can elucidate underlying neurocognitive effects. We tested methylphenidate effects relative to placebo in functional magnetic resonance imaging (fMRI) during three disorder-relevant tasks in medication-naïve ADHD adolescents. In addition, we conducted a systematic review and meta-analysis of the fMRI findings of acute stimulant effects on ADHD brain function.
Methods
The fMRI study compared 20 adolescents with ADHD under either placebo or methylphenidate in a randomized controlled trial while performing stop, working memory, and time discrimination tasks. The meta-analysis was conducted searching PubMed, ScienceDirect, Web of Knowledge, Google Scholar, and Scopus databases. Peak coordinates of clusters of significant effects of stimulant medication relative to placebo or off medication were extracted for each study.
Results
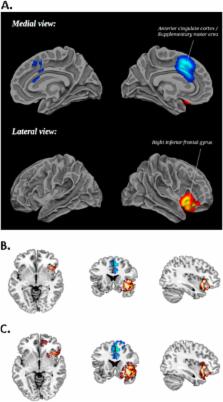
The fMRI analysis showed that methylphenidate significantly enhanced activation in bilateral inferior frontal cortex (IFC)/insula during inhibition and time discrimination but had no effect on working memory networks. The meta-analysis, including 14 fMRI datasets and 212 children with ADHD, showed that stimulants most consistently enhanced right IFC/insula activation, which also remained for a subgroup analysis of methylphenidate effects alone. A more lenient threshold also revealed increased putamen activation.
Related collections
Most cited references77
- Record: found
- Abstract: found
- Article: not found
Using Support Vector Machine to identify imaging biomarkers of neurological and psychiatric disease: a critical review.
- Record: found
- Abstract: found
- Article: not found
Voxel-wise meta-analysis of grey matter changes in obsessive-compulsive disorder.
- Record: found
- Abstract: not found
- Article: not found
