- Record: found
- Abstract: found
- Article: found
Mouse REC114 is essential for meiotic DNA double-strand break formation and forms a complex with MEI4
Read this article at
Abstract
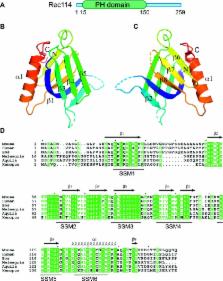
Mouse REC114 is essential for meiotic DNA double-strand break formation and forms a complex with IHO1. Its N-terminal region forms a Pleckstrin homology domain, while its C-terminal region is interacting with MEI4.
Abstract
Programmed formation of DNA double-strand breaks (DSBs) initiates the meiotic homologous recombination pathway. This pathway is essential for proper chromosome segregation at the first meiotic division and fertility. Meiotic DSBs are catalyzed by Spo11. Several other proteins are essential for meiotic DSB formation, including three evolutionarily conserved proteins first identified in Saccharomyces cerevisiae (Mer2, Mei4, and Rec114). These three S. cerevisiae proteins and their mouse orthologs (IHO1, MEI4, and REC114) co-localize on the axes of meiotic chromosomes, and mouse IHO1 and MEI4 are essential for meiotic DSB formation. Here, we show that mouse Rec114 is required for meiotic DSB formation. Moreover, MEI4 forms a complex with REC114 and IHO1 in mouse spermatocytes, consistent with cytological observations. We then demonstrated in vitro the formation of a stable complex between REC114 C-terminal domain and MEI4 N-terminal domain. We further determine the structure of the REC114 N-terminal domain that revealed similarity with Pleckstrin homology domains. These analyses provide direct insights into the architecture of these essential components of the meiotic DSB machinery.
Related collections
Most cited references47
- Record: found
- Abstract: not found
- Article: not found
A drying-down technique for the spreading of mammalian meiocytes from the male and female germline.
- Record: found
- Abstract: found
- Article: not found
The mouse Spo11 gene is required for meiotic chromosome synapsis.
- Record: found
- Abstract: found
- Article: not found