- Record: found
- Abstract: found
- Article: found
Factors associated with choice of biologic among children with Juvenile Idiopathic Arthritis: results from two UK paediatric biologic registers
Read this article at
Abstract
Objective. The objectives of this study were to describe patients starting first-line biologics for JIA, to describe characteristics over time among patients starting etanercept, and to describe patterns of second biologic prescribing.
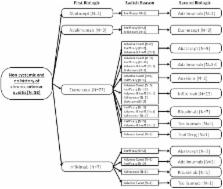
Methods. The British Society for Paediatric and Adolescent Rheumatology Etanercept Cohort Study, and the Biologics for Children with Rheumatic Diseases study are ongoing prospective observational cohorts, collecting data on patients starting biologic therapy for JIA. Patients registered from 1 January 2010 starting their first biologic were compared between therapies. Patients starting etanercept before 2010 were included to analyse changes in etanercept prescribing. The pathway of patients starting a second biologic was recorded in all patients.
Results. To 26 August 2014, 931 patients were recruited starting a first-line biologic (142 Biologics for Children with Rheumatic Diseases; 789 British Society for Paediatric and Adolescent Rheumatology Etanercept Cohort Study). From 2010, patients with systemic JIA (sJIA) were almost exclusively prescribed anakinra or tocilizumab. Choice between anti-TNF therapies was largely driven by history of chronic anterior uveitis (CAU). When investigating trends in patients starting etanercept over time, disease duration at etanercept start, patients with sJIA, a history of CAU, and those who received concomitant oral corticosteroids decreased over time. Patients who started a second biologic from 1 January 2010 showed a similar stratification.
Conclusion. Although etanercept remains the most common biologic prescribed for JIA, there has been a clear shift towards the use of alternative biologics, largely driven by disease subtype and history of CAU. This channelling of children towards specific therapies should be considered carefully in future studies and in clinical guidelines and ongoing research.
Related collections
Most cited references20
- Record: found
- Abstract: found
- Article: not found
EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs

- Record: found
- Abstract: found
- Article: not found