- Record: found
- Abstract: found
- Article: found
Enhanced detoxification of Cr 6+ by Shewanella oneidensis via adsorption on spherical and flower-like manganese ferrite nanostructures†

Read this article at
Abstract
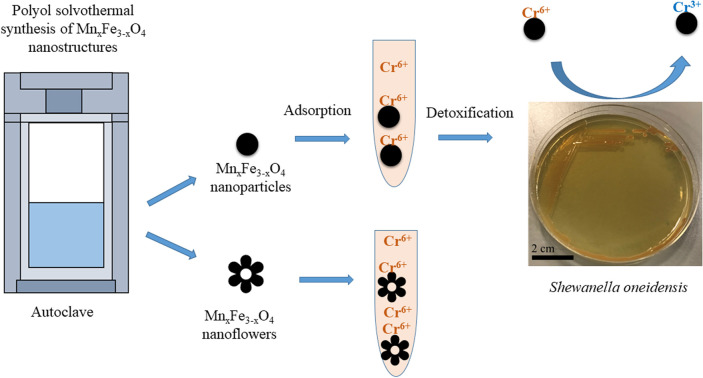
Maximizing the safe removal of hexavalent chromium (Cr 6+) from waste streams is an increasing demand due to the environmental, economic and health benefits. The integrated adsorption and bio-reduction method can be applied for the elimination of the highly toxic Cr 6+ and its detoxification. This work describes a synthetic method for achieving the best chemical composition of spherical and flower-like manganese ferrite (Mn x Fe 3− x O 4) nanostructures (NS) for Cr 6+ adsorption. We selected NS with the highest adsorption performance to study its efficiency in the extracellular reduction of Cr 6+ into a trivalent state (Cr 3+) by Shewanella oneidensis ( S. oneidensis) MR-1. Mn x Fe 3− x O 4 NS were prepared by a polyol solvothermal synthesis process. They were characterised by powder X-ray diffraction (XRD), transmission electron microscopy (TEM), X-ray photoelectron spectrometry (XPS), dynamic light scattering (DLS) and Fourier transform-infrared (FTIR) spectroscopy. The elemental composition of Mn x Fe 3− x O 4 was evaluated by inductively coupled plasma atomic emission spectroscopy. Our results reveal that the oxidation state of the manganese precursor significantly affects the Cr 6+ adsorption efficiency of Mn x Fe 3− x O 4 NS. The best adsorption capacity for Cr 6+ is 16.8 ± 1.6 mg Cr 6+/g by the spherical Mn 0.2 2+Fe 2.8 3+O 4 nanoparticles at pH 7, which is 1.4 times higher than that of Mn 0.8Fe 2.2O 4 nanoflowers. This was attributed to the relative excess of divalent manganese in Mn 0.2 2+Fe 2.8 3+O 4 based on our XPS analysis. The lethal concentration of Cr 6+ for S. oneidensis MR-1 was 60 mg L −1 (determined by flow cytometry). The addition of Mn 0.2 2+Fe 2.8 3+O 4 nanoparticles to S. oneidensis MR-1 enhanced the bio-reduction of Cr 6+ 2.66 times compared to the presence of the bacteria alone. This work provides a cost-effective method for the removal of Cr 6+ with a minimum amount of sludge production.
Abstract
An enhancement strategy for the bio-reduction of Cr 6+ in the presence of manganese ferrite nanostructures using Shewanella oneidensis.
Related collections
Most cited references1
- Record: found
- Abstract: not found
- Book: not found